
47 Molter Street
Cranston, Rhode Island 02910-1032
401-781-6100 • info@technic.com • www.technic.com
Application Notes
Physical Properties of Electrodeposited Nickel
The mechanical properties of electrodeposited nickel are influenced by the operating variables - pH,
temperature, and cathode current density. The constituents of the solution, if their concentrations are not
kept within specified limits and relatively small amounts of metallic impurities, may also affect mechanical
properties. The properties are interrelated and steps taken to increase the hardness of the deposit usually
increase its strength and lower its ductility. The refinement of crystal structure, for example by the use of
organic addition agents, is accompanied by increased hardness and tensile strength, and reduced
ductility.
The influence of operating variables on some of the properties of nickel deposited from Watts and
conventional nickel sulfamate solutions are related in Tables 1 and 2. Deposits from these types of nickel
baths are affected differently by the same variables. For example, in the Watts, solution tensile strength is
relatively independent of plating solution temperature, pH and cathode current density; it increases with
increasing nickel and chloride in solution. In the sulfamate solution, tensile strength decreases with
increasing temperature to 50 C, increases with increasing pH, and decreases with increasing cathode
current density, it decreases slightly with increasing nickel and chloride in solution. The operating
variables, as well as the specific constituents, affect the properties of electrodeposited nickel. Summaries
of typical physical properties of deposits from various nickel bath formulations are included in Table 3.
In addition, the mechanical properties, especially the percent elongation or ductility, are affected by the
thickness of the electrodeposited nickel used in determining the properties. The ductility increases with
increasing nickel thickness up to about 250 micrometers after which it becomes relatively constant. This
was shown in the classic work by Zentner, Brenner and Jennings (1952) for deposits from Watts solutions
and is true for nickel deposits from sulfamate solutions. Mechanical testing should be done at the
thickness of interest even though it may be more convenient to test thick deposits. (Note: ASTM B489-85
specifies metal deposit thickness of 25 to 40 micrometers, or 1.0 to 1.8 mils thick)
The properties of nickel electrodeposited from sulfamate solutions can be affected by uncontrolled anode
behavior, which results in the oxidation of the sulfamate anion. The oxidation products can lower the
internal stress and increase the sulfur content of the deposits. The extent to which these changes in
internal stress and sulfur content affect the ultimate tensile strength and per cent elongation of sulfamate
nickel electrodeposits has been studied (Chart, 1977). Nickel sulfamate deposits with tensile internal
stress were obtained from conventional solutions; the stress was stable at 50MPa (7,252 psi). (The
solution contained 70 g/l of nickel metal as the sulfamate, 0.1 g/l of chloride added as nickel chloride
hexahydrate, and 35 g/l boric acid. The pH was 4.0, temperature 60 C, and cathode current density 540
A/m
2
(58 ASF); the bath was operated with air agitation.)
After the tensively stressed deposits were prepared over a range of thicknesses, similar compressively
stressed deposits were prepared by including a platinum foil anode in the circuit and passing 1 to 2 per
cent of the total current through the auxiliary anode; the current density on the auxiliary anode was 2.7
A/m
2
. This procedure gave deposits with an internal stress that was 71 MPa in compression.
The ultimate tensile strength varies with nickel thickness but becomes stable above 250 micrometers. The
strength of the compressively stressed deposits is greater than that of the tensively stressed deposits.
Annealing at 371 C for 2 hours lowers the tensile strength of the compressively stressed and tensively
stressed deposits to approximately equal values. Annealing at the higher temperature lowers the tensile
strength even further, but the decrease is significantly greater in the case of the compressively stressed
deposits.

The work established that the oxidation products formed at an insoluble platinum anode in sulfamate
solutions lower internal stress and result in the codeposition of sulfur. The codeposition of small amounts
of sulfur affects the mechanical properties of electroformed nickel especially at high temperatures. It is
important to control the anode behavior to achieve consistent results in electroforming from sulfamate
solutions.
Internal Stress
The control of internal stress is extremely important in electroforming. Internal stress refers to forces
created within an electrodeposit because of the electro crystallization process and/or the codeposition of
impurities such as hydrogen, sulfur and other elements. The forces are either tensile (contractile) or
compressive (expansive) in nature; rarely are electrodeposits free of some degree of internal stress.
Excessive tensile or compressive stress can cause the following problems: 1) distortion of the electroform
when it is separated from the mandrel; 2) difficulty of separating the electroform from the mandrel; 3)
curling, peeling or separation of the electroform prematurely from the mandrel; and 4) buckling and
blistering of the deposit that is usually indicative of high compressive stress.
Internal stress is influenced by the nature and composition of the nickel plating solution (see Table 4).
The all-chloride solution produces deposits with the highest internal stresses. Nickel sulfamate solutions
without chlorides produce deposits with the lowest internal stresses. As discussed, organic additives can
be used to control internal stress of electrodeposited nickel, but
these additives invariably introduce sulfur they must be used with caution and due consideration. Sulfur
codeposited with nickel increases its hardness and strength, and reduces ductility. Sulfur affects the high
temperature properties adversely, and nickel deposits with sulfur cannot be heated above 200 C without
becoming embrittled. The codeposition of small amounts of manganese has been shown to prevent
embrittlement of sulfur-containing nickel electrodeposits and allows heating above that temperature. The
concentrated nickel sulfamate process discussed above can be operated at high current densities to yield
deposits with very low or zero internal stresses, the techniques being shown to be effective with nickel as
well as nickel-cobalt alloy electroforming. Internal stress is controlled by specifying the electrolyte and
maintaining its purity, and by using specific organic addition agents in controlled concentrations. Control
of current density and the other operating variables is also important.
Ductility
There are two commonly used test methods used to evaluate the ductility of electrodeposits. The first of
these has its origin for the control of decorative bright or semi bright nickel plating where full bright
deposits tended to become brittle due to excessive sulfur codeposition. The test (ISO 1456 or ASTM
B489-85) is conveniently carried out by cutting a test strip 150 mm long and 10 mm wide (6 x ½ inches)
from a larger sheet of brass or cold rolled steel, which has been plated with 25 – 40 micrometers (1 to 1.8
mils) of nickel deposit. The test strip is bent, with the plated surface outermost, through 180 degrees over
a mandrel of 0.203 inches (13/64 inch) until the two ends of the test strip are parallel. If no cracks pass
completely across the convex surface when examined a 10-x magnification, the plating has an elongation
greater than 8 percent. (Note: See Table 5 for the relationship between the diameter of bend radius and
percent elongation)
The second, less commonly used method, is uniaxial tension or pull testing. Although this test can
provide both measurements of internal stress and ductility, the preparation of foil test specimens is
demanding and the test equipment is generally not available in any but the largest companies. The
results from the uniaxial pull testing of foils cannot be compared directly with the same deposits as
measured by the bend test method. Ductility measurements based on the bend test include the base
substrate as an integral part of the test. In fact, it would be assumed that bend test results done in
separate facilities using different substrate alloys and or thicknesses would produce different results.
Hence, the unspecified range of the specification call out in Mil QQ-N-290A and ASTM B489-85 requiring
greater than 8 percent elongation. Although it is possible to produce foils that will pass the 8 percent
minimum requirement, typical results, especially those baths containing additives are lower. As is the

case with the bend test method, foils of differing thickness and produced on different substrates will have
different elongations. Note: Table 7 Compares of elongation test results made from the same nickel
electrolytes and measured with different test procedures.
The ductility shows greater variation with thickness than does the ultimate tensile strength. The ductility is
greater for the tensively stressed deposits than for the compressive ones in the as-plated condition.
Annealing at 371 C increases the ductility of both types of deposits. Annealing at 760 C increases the
ductility of the tensively stressed deposits, but lowers the ductility of the compressively stressed ones to
values below the as-plated ones. The measurements of ultimate tensile strength and percent elongation
(ductility) were made by standard uniaxial tension testing. The deposits were also analyzed chemically.
The tensively stressed deposits contained less than 1 part per million sulfur, whereas the compressive
deposits contained about 40 parts per million sulfur. Metallographic and electron microprobe analyses
conducted after annealing showed brittle failure in compressive deposits heated at 760 C, as well as high
sulfur (380 to 500 ppm) contents in grain boundaries.
Hardness
The hardness of a metal is not a function of a single property of the material, but rather a combination of
properties that we refer to as hardness. Although hardness is not a fundamental property of materials, it
is useful in general metallurgy as a control test that may be correlated with other properties that are more
difficult or expensive to determine. In the case of electrodeposits, published evidence would indicate that
hardness values cannot be well correlated with the tensile properties of the same deposit. The
correlation between the hardness of a deposit and its brightness has been systematically investigated
and concludes that there is only a casual relationship and the exceptions to it are more numerous and
significant than the apparent increase in brightness with increase in hardness. The hardness of a metal is
indicative of its resistance to abrasion and ware, i.e., the higher the hardness the higher the resistance.
Although the correlation may not be exact, this is one of the best reasons for being interested in the
hardness of electrodeposited metals. The hardness of electrodeposits is influenced by the conditions of
deposition, both the solution composition and the conditions for deposition. This is particularly true for
nickel and indicates the care that should be taken during the plating operation if the hardness of the
deposit is to be controlled.
The most common method to determine hardness of electrodeposits is based on resistance to
penetration by a loaded indenter. The hardness numbers obtained with any of these indenters are
dependant upon the load applied the friction relationship between the indenter and the metal, the
geometry of the indenter, the work hardening of the sample during mounting, etc. These many variables
become particularly important when the size of the indent is small as is the typical case with
electrodeposited metal coatings. It is important, therefore, to specify the technique and conditions
whenever specifying hardness values for electrodeposited coatings. If the surface of the coating is too
rough for observation and measurement of the indent, or if the deposit is too thin to avoid the anvil effect
(less than fifteen times the length of the indent), it is necessary to prepare a metallographic cross section
of the deposit. (Note: See the procedure for the preparation of samples for hardness analysis)
Table 4 relates the thickness of deposits, which are required to avoid the anvil effect when measuring the
hardness of deposits for various loads with a Knoop indenter for metals of several hardness’s. When the
hardness of a cross section is measured, it is necessary that the short diagonal of the Knoop indent fall
within the coating with at least one half the width of the indent to spare. If this condition is not met, the
load being used must be reduced appropriately. Illustrative data for selected nickel solutions is given in
figure 1.
Grain Size
The ductility, the tensile strength and other properties of metals are often influenced by grain size.
However, there are very few useful correlations between the grain size of a deposit and other physical
properties. There are some results, which indicate that, at least in a general way, the tensile strength of

electroplated deposits increases with increasing hardness, unfortunately there are also numerous
exceptions. There is reasonably good evidence that ductility and grain size are not uniformly related in
electrodeposits.
It has been suggested that the brightness of a deposit is intimately related to the grain size, i.e., the
smaller the grain size the brighter the deposit. At least in the case of nickel it has been established that
there is no correlation between grain size and brightness. Brightness is a surface effect and not a bulk
property of the deposit. Studies with the electron microscope reveal that for brightness the shape and
packing of grains is more important than the absolute size.
References:
Di Bari, G.A., “Electroforming”, Electroplating Engineering Handbook – Fourth Edition,
Edited by L.J. Durney, Van Nostrand Reinhold Company Inc New York 1984.
Zetner, J., Brenner, A. and Jennings, C.W., “Physical Properties of Electrodeposited Metals Part I Nickel”,
Plating 39 (8) 865-927 (1952).
Safranek, W.H., “The Properties of Electrodeposited Metals and Alloys – A Handbook”, Second Edition,
American Electroplaters and Surface Finishers Society, Orlando Florida, 1986.
“Practical Nickel Plating” – Second Edition, International Nickel Company Inc., New York, N.Y. 1959
ASTM B 489-85 (Reapproved 1998) “Standard Practice for Bend Test for Ductility of Electrodeposited
and Autocatalytically Deposited Metal Coatings on Metal” ASTM 100 Barr Harbor Drive, West
Conshohocken, PA 19428
Rev. Initial 8/22/03

Table 1 - Variables Which Affect Physical Properties of Nickel Sulfamate Deposits
Property
Operational
Solution Composition
Tensile
Strength
Decreases with increasing temperature to 49C (120F)
then increases slowly with further temperature increase
Increases with increasing pH
Decreases with increasing current density
Decreases slightly with increasing nickel content
Elongation
Decreases as the temperature varies in either direction from
43C (110 F)
Decreases with increasing pH
Increases moderately with increasing current density
Increases slightly with increasing nickel content
Increases slightly with increasing chloride content
Hardness
Increases with increasing temperature within specified operating
range
Increases with increasing solution pH
Reaches a minimum at about 1300 A/m
2
(120 A/ft
2
)
Decreases slightly with increasing nickel concentration
Decreases slightly with increasing chloride concentration
Internal Stress
Decreases with increasing solution temperature
Reaches a minimum at pH 4.0 to 4.2
Increases with increasing current density
Relatively independent of nickel concentration within specified
operating range
Increases significantly with increasing chloride content
Table 2 - Variables Which Affect Physical Properties of Watts Nickel Deposits
Property
Operational
Solution Composition
Tensile
Strength
Relatively independent of plating solution temperature within specified range
Relatively independent of pH variation within specified range
Relatively independent of changes in cathode current density
Increases with increasing nickel
content
Increases with increasing chloride
content
Elongation
Increases with temperature to 55C (130F) followed by slight decrease at higher temperature
Relatively independent of pH within specified range
Decreases with increasing nickel
content
Hardness
Decreases with temperature rise to 55C (130F) but increases with higher temperature
Decreases significantly with increasing cathode current density to 540 A/m
2
(50 A/ft
2
) at higher current
densities hardness increases with increasing current density
Increases with increasing nickel
concentration
Increases with increasing chloride
concentration
Internal
Stress
Relatively independent of solution temperature
Relatively independent of pH within specified range
Decreases slightly, then increases with increasing current density
Increases slightly with increasing
nickel content
Increases significantly with increasing
chloride content

Table 3 - Nickel Electroplating Solutions
And Typical Properties of Deposits
Electrolyte Composition
1
Watts Nickel
Standard
Sulfamate
Conc. Sulfamate
Typical Semi-
Bright
2
NiSO
4
.
6H
2
O
225-300
300
Ni (SO
3
NH
2
)
2
.
4H
2
O
315-450
500-650
NiCl
2
.
6H
2
O
37-53
0-22
0-22
35
H
3
BO
3
30-45
30-45
30-45
45
Operating Conditions
Temperature C
44-66
32-60
32-60
54
Agitation
Air-Mechanical
Air-Mechanical
Air-Mechanical
Air-Mechanical
Current A/dm
2
3-11
0.5-32
0.5-32
3.0 - 10
Anodes
Nickel
Nickel
Nickel
Nickel
pH
3.0-4.2
3.5-4.5
3.5-4.5
3.5-4.5
Mechanical Properties
Tensile MPa
345-485
415-620
415-620
-
Elongation %
15-25
10-25
10-25
8-20
Vickers 100g
130-200
170-230
170-230
300-400
Internal Stress MPa
125-185 tensile
0-85 tensile
0-55 tensile
3
35-150
Notes:
1. Surfactant agents formulated for nickel plating are added to control pitting
2. Proprietary additives are required for semi-bright deposits
3. Near zero stress may be obtained at various combinations of current density and temperature.
4. Reference: Typical full bright deposit properties, elongation 2-5%, Vickers Hardness 100 gram 600-800, Internal
Stress MPa 12-25 compressive
Table 4 - Typical Internal Stress for Nickel Plating Solutions
Nickel Electrolyte
Internal Stress MPa
Watts
110-210
Watts with Hydrogen Peroxide
275 or more
All Chloride
205-310
Fluoborate
100-175
Fluoborate with Hydrogen Peroxide
100-175
Sulfamate, no chloride
0-55
Sulfamate, with chloride
55-85
All Sulfate
110-140
Notes: 1.0 MPa equals 145.04 psi
1 pascal = Newton square meter = 0.00015 psi
1.0 psi = 6,895 Pascal
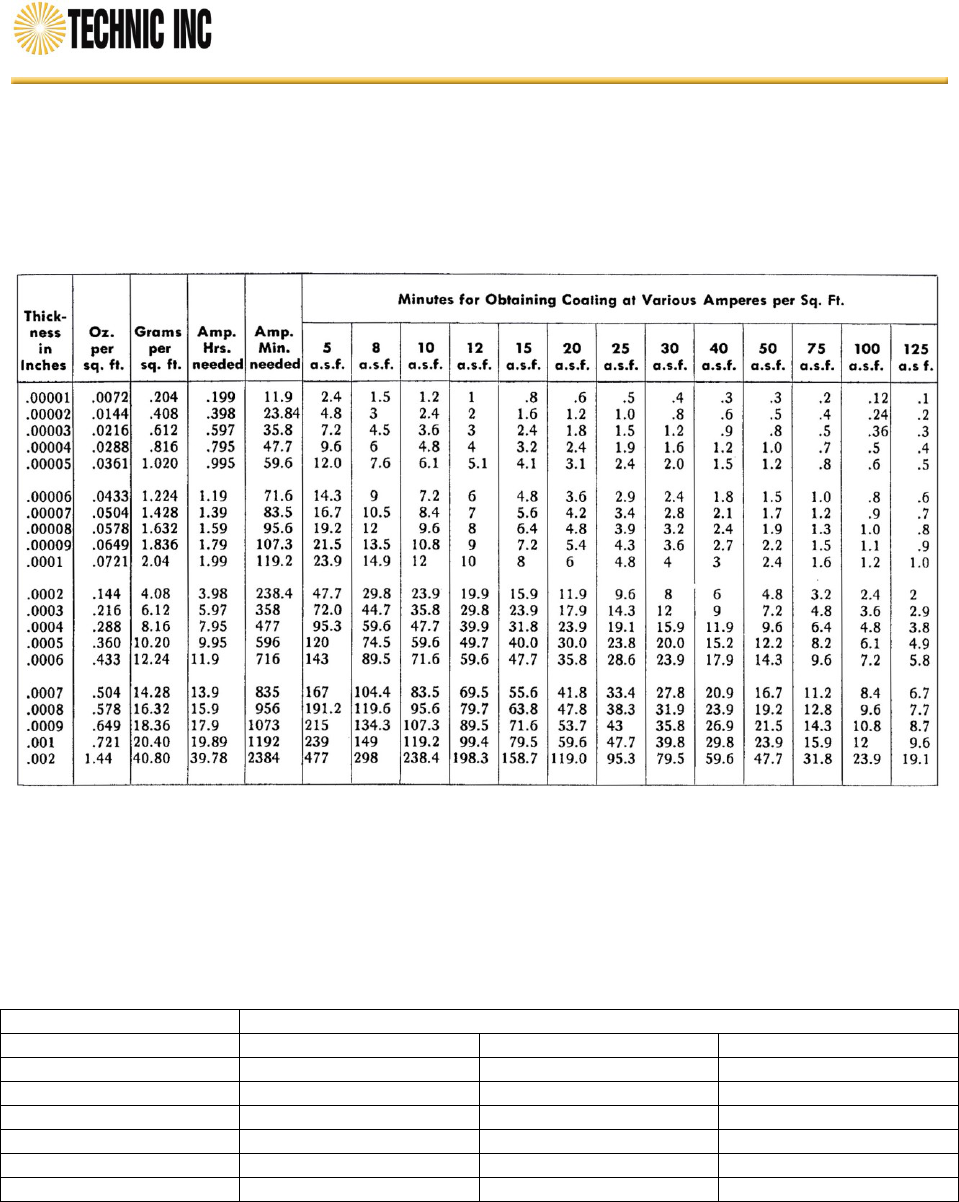
Table 5 - Plating Rates for Electrodeposited Nickel
(Based on 96.5% Cathode Efficiency)
Table 6 - Minimum Deposit Thicknesses to Avoid Anvil Effect in Hardness
Measurements with Knoop Indenter
Knoop Hardness
Minimum Thickness in Mils
25 gram Load
100 gram Load
200 gram Load
100
1.1
2.2
3.0
300
0.6
1.2
1.8
500
0.5
1.0
1.4
700
0.4
0.8
1.2
900
0.4
0.7
1.0
1100
0.3
0.7
0.9
Notes: Refer to Table 5 to calculate the time at current to produce the required minimum thickness for nickel to avoid
the anvil effect.


Table 7 – Nickel Ductility as Measured with Different Test Methods
Process
Bend Test
1
Uniaxial Pull
2
Control
Electrolyze
JB – 100
Control
Electrolyze
JB - 100
Sulfamate S
22%
22%
22%
2-3%
5-9%
2-5%
High Throw
22%
22%
22%
4-6%
4-9%
3-5
Notes:
1. Control, is the “as is” make up for each bath per the respective technical data sheet, except without
proprietary additives.
2. Electrolyzed, is the control bath make up with carbon, peroxide and electrolytic dummy cycle following make
up and prior to sample preparation.
3. JB-100 is the electrolyzed bath with the recommended addition of JB-100 per the respective technical data
sheet.
Figure 1 Knoop Hardness Values for Selected Electrolytic Nickel Deposits
Notes:
1. NS is Techni Nickel Sulfamate S, HT-2 is Techni Nickel Hi-Throw 2 and all samples are plated to a thickness
of 3 mils.
2. 100-gram and 200-gram load values on the same sample vary slightly; use the load value recommended by
your specification call out.
3. Control, is the “as is” make up for each bath per the respective technical data sheet, except without
proprietary additives.
4. Electrolyzed, is the control bath make up with carbon, peroxide and electrolytic dummy cycle following make
up and prior to sample preparation.
5. JB-100 is the electrolyzed bath with the recommended addition of JB-100 per the respective technical data
sheet.
0
100
200
300
400
500
600
Control
Electrolyzed
JB 100
Knoop Hardness * Gram Load
Nickel Electrolyte Condition
Electrolytic Nickel Hardness
NS 100 g
NS 200 g

Table 6 - Bend Test for Nickel Ductility
% Elongation
Mandrel Dia. inches
% Elongation
Mandrel Dia. inches
1
1.782
51
0.017
2
0.882
52
0.017
3
0.582
53
0.016
4
0.432
54
0.015
5
0.342
55
0.015
6
0.282
56
0.014
7
0.239
57
0.014
8
0.207
58
0.013
9
0.182
59
0.013
10
0.162
60
0.012
11
0.146
61
0.012
12
0.132
62
0.011
13
0.120
63
0.011
14
0.111
64
0.010
15
0.102
65
0.010
16
0.095
66
0.009
17
0.088
67
0.009
18
0.082
68
0.008
19
0.077
69
0.008
20
0.072
70
0.008
21
0.068
71
0.007
22
0.064
72
0.007
23
0.060
73
0.007
24
0.057
74
0.006
25
0.054
75
0.006
26
0.051
76
0.006
27
0.049
77
0.005
28
0.046
78
0.005
29
0.044
79
0.005
30
0.042
80
0.005
31
0.040
81
0.004
32
0.038
82
0.004
33
0.037
83
0.004
34
0.035
84
0.003
35
0.033
85
0.003
36
0.032
86
0.003
37
0.031
87
0.003
38
0.029
88
0.002
39
0.028
89
0.002
40
0.027
90
0.002
41
0.026
91
0.002
42
0.025
92
0.002
43
0.024
93
0.001
44
0.023
94
0.001
45
0.022
95
0.001
46
0.021
96
0.001
47
0.020
97
0.001
48
0.020
98
0.000
49
0.019
99
0.000
50
0.018
100
0.000
Values are for a 0.017" Hull Cell panel substrate and a deposit of 0.001"
For other dimensions use E = 100T/(D +T), E = % Elongation, D = Mandrel diameter and
T = Thickness of substrate plus deposit in inches

