
Reporting Guide to Monitor Testing

2
December 2021
3
Reporting Guide to Monitor Testing
Contents
1. INTRODUCTION................................................................................................................................................................ 3
2. GENERATE A LAB RESULT REPORT (LRR) IN ADAMS......................................................................................... 5
3. GENERATE A SAMPLE COLLECTION REPORT (SCR) IN ADAMS .................................................................... 10
4. IDENTIFY AND CORRECT MISTAKES IN ADAMS REPORTS ............................................................................. 13
5. MONITOR DCF ENTRY IN ADAMS ............................................................................................................................. 17
6. TDSSA MONITORING .................................................................................................................................................... 20
7. CONTACT ......................................................................................................................................................................... 20

3
December 2021
3
1. Introduction
On 2 October 2014, the World Anti-Doping Agency (WADA) published the Technical Document
for Sport Specific Analysis (TDSSA), which is intended to ensure that Prohibited Substances
and/or Prohibited Methods within the scope of the TDSSA and other tools that support the
detection of Prohibited Substances and/or identify the Use of Prohibited Methods such as the
Athlete Biological Passport (ABP) are subject to an appropriate and consistent level of use,
analysis and adoption by all Anti-Doping Organizations (ADOs) that conduct testing in the relevant
sports and disciplines. Compliance with the TDSSA is mandatory under article 4.2.4 of the 2021
International Standard for Testing and Investigations (ISTI); and, since 1 January 2016 WADA
has been monitoring implementation of the TDSSA closely.
On 1 June 2016, following a decision by WADA’s Executive Committee, it became mandatory for
ADOs to enter all Doping Control Forms (DCFs) into the Anti-Doping Administration and
Management System (ADAMS) within twenty-one days of the date of sample collection.
As both TDSSA implementation and entry of DCFs into ADAMS are mandatory, WADA monitors
them closely from a Code compliance perspective. Failure to comply with these requirements can
result in a declaration of non-compliance.
WADA developed this new Reporting Guide to Monitor Testing in order to assist ADOs with these
compliance requirements. The Guide provides step by step instructions to:
generate relevant reports in ADAMS;
cross check the data and;
take the necessary action to eliminate any data entry errors,
so that ADOs can monitor their compliance with the TDSSA and DCF entry based on accurate
testing data.
There are various ways to determine the number of tests and analyses from the ADAMS reports.
This Guide illustrates a basic way to perform the exercise; using vlookup in Excel to extract the
relevant figures based on the principles outlined in this Guide.
Internet Explorer 11 and Microsoft Excel 2016 were used to create the screenshots and the
instructions in this Guide. The procedures may be slightly different on other web browsers and
different versions of Microsoft Excel.
All ADAMS templates recommended in this Guide are created in the current ADAMS system and
require some manual handling of data to identify the missing or wrong information, if any, and to
extract the relevant figures for compliance monitoring.

4
December 2021
3
Overview of the Monitoring Process

5
December 2021
3
2. Generate a Lab Result Report (LRR) in
ADAMS
The LRR retrieves information from the sample analysis results that are submitted by Laboratories
in ADAMS.
1. Choose the Lab Result Report in the Reports module on the ADAMS main page.
2. Open the existing template created by WADA.
a) Click open at the top right corner of the LRR page.
b) In the pop-up
1
Open report window:
Select WADA-AMA in the Organization field;
Choose LRR DCF Entry Monitor in the Report group field;
The name of the template LRR DCF Entry Monitor will appear in the Report
name field.
1
ADAMS requires pop-ups to operate properly. Please ensure that your browser allows pop-ups coming from https://adams.wada-
ama.org/adams/

6
December 2021
3
c) Click open to open the template.
3. Click select to choose your organization in the Testing Authority field and then click
save.
4. Specify the Collection Date range to define the scope of data to be included in the
report. This can be done by either typing a date in the from/to fields or selecting
from the pop-up calendar.
Testing Authority

7
December 2021
3
5. Click view report at the top or bottom of the LRR page to launch the report and start
retrieving data.
6. A Report has been launched window will pop up. Click on go to My Reports to
proceed to My Reports page.
7. Open and view the completed LRR.
a) Use the Refresh button to refresh My Reports page if the report is not yet completed
after the page is open.
b) Click on the Completed status icon and the report will open as a CSV file.
8. Open the report as a CSV file and convert it to an Excel spreadsheet as explained
in section ‘Convert the CSV file to an Excel spreadsheet’ in this chapter.
Video on how to generate the Lab Result Report (LRR)
.
8
December 2021
3
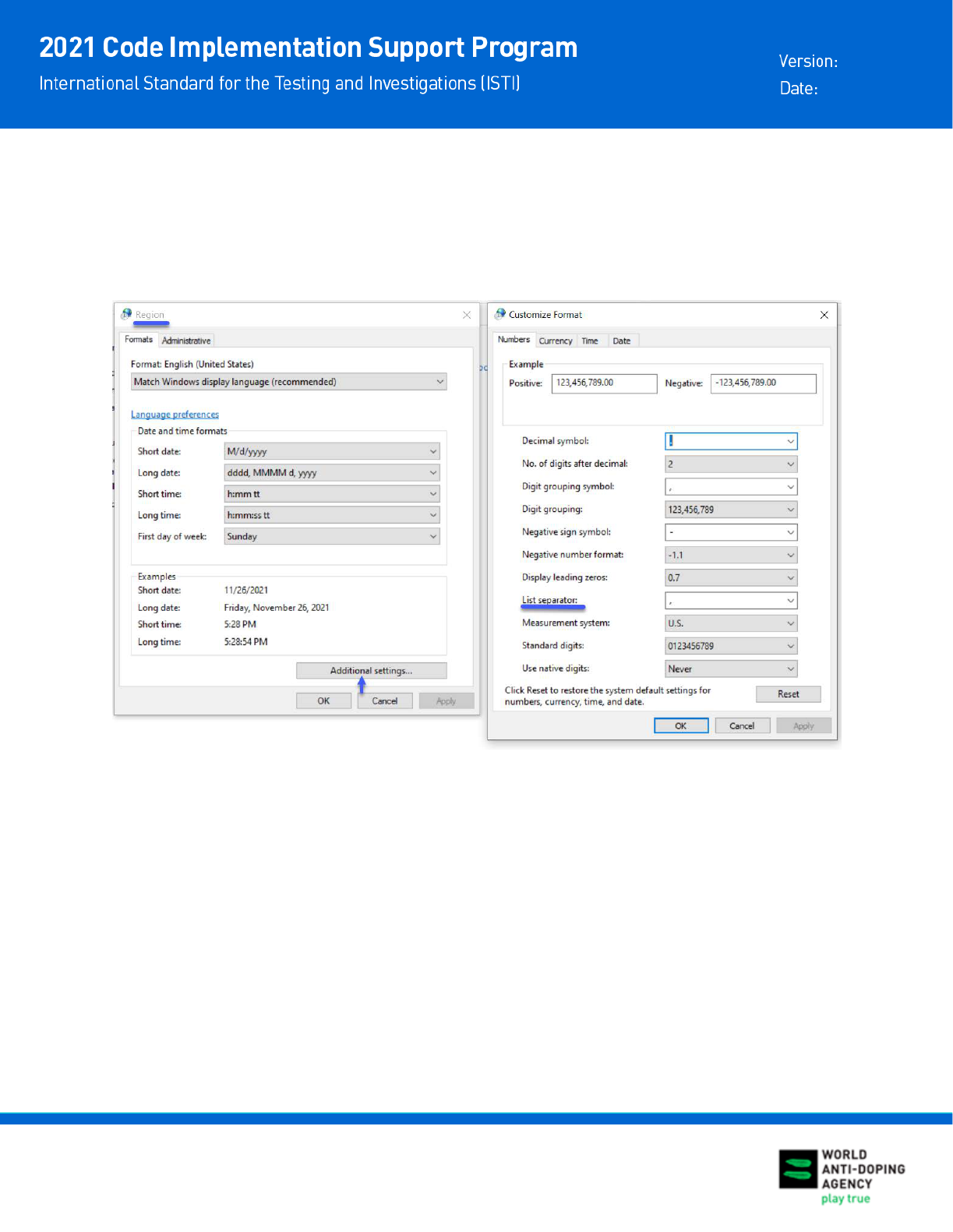
DATA CLUSTER
If the data is clustered in the first column of each row in the CSV file, you will need to change the
List separator setting in your computer. Go to Control Panel-Region-Additional settings. Set List
separator value to comma “,” as shown in the screenshot below. Open the csv file again and it
will look as a properly formatted spreadsheet.
CONVERT THE CSV FILE TO AN EXCEL SPREADSHEET:
a) When the CSV file is opened, click the File tab at the top left corner of the Excel
window and select Save As.
b) Click the drop-down menu to the right of Save as type and choose the Excel
Workbook (*.xlsx) option at the top of the list.
c) Type a name for the new file in the File name field, then click on Save.
d) It is suggested to add the date when the report is generated in the name of the file to
track data extracted from ADAMS on different days.

9
December 2021
3

10
December 2021
3
3. Generate a Sample Collection Report (SCR)
in ADAMS
1. Choose the Sample Collection Report in the Reports module on the ADAMS main
page.
2. Open the existing template created by WADA:
a) Click open at the top right corner of the SCR page.
b) In the pop-up Open report window:
Select WADA-AMA in the Organization field;
Choose SCR ADO Statistics Report in the Report group field;
The name of the template SCR ADO Statistics Report will appear in the
Report name field;
c) Click open to open the template.

11
December 2021
3
3. Click select to choose your organization in the Testing Authority field and then click
save.
4. Specify the Collection Date range to define the scope of data to be included in the
report. This can be done by either typing a date in the from/to fields or selecting
from the pop-up calendar.
5. Click view report at the top or bottom of the SCR page to launch the report and start
retrieving data.
6. A Report has been launched window will pop up. Click on go to My Reports to
proceed to My Reports page.

12
December 2021
3
7. Open and view the completed SCR.
a) Use the Refresh button to refresh My Reports page if the report is not yet completed
after the page is opened.
b) Clicking on the Completed status icon will open the report as a CSV file.
8. Open the report as a CSV file and convert it to an Excel spreadsheet as explained
in section ‘Convert the CSV file to an Excel spreadsheet’ of chapter 2.
Video on how to generate the Sample Collection Report (SCR)
.

13
December 2021
3
4. Identify and Correct Mistakes in ADAMS
Reports
ADOs are recommended to generate a LRR and a SCR to verify the accuracy of the analysis data
related to their organization on a regular basis (e.g., monthly) so that any data entry errors are
corrected in a timely fashion. Such checking and correcting exercise is also important to ensure
that ADOs’ testing data are accurately reflected in the global Anti-Doping Testing Figures Reports.
The LRR generated by following the instructions in Section 2 of this Guide contains the results of
all urine and blood samples reported by the laboratories in ADAMS with your organization as the
Testing Authority (TA). Athlete Biological Passport (ABP) blood sample results are not included
in this LRR and those results can be extracted separately from a biological result report in ADAMS
if needed.
The SCR generated by following the instructions in Section 3 of this Guide contains all urine and
blood samples for which your organization is recorded as the TA.
ADOs should verify the sample codes in the two reports against one another.
Video on How to Identify Mistakes Using the LRR and SCR
.
For a small number of samples, an easy way could be sorting the sample codes from smallest to
largest in both reports, copy them to two columns next to each other in one Excel worksheet and
then compare the codes in the same row. See the illustration below.

14
December 2021
3
For a large number of samples, it is recommended to use the “vlookup” formula in Excel. See the
following video which demonstrates how to use this formula to verify if the LRR samples are in
the SCR: https://youtu.be/6BvPN5lMs7U
The four arguments in the vlookup fomula are:
1. Lookup_value: the cell to the left of the formula, containing the sample codes in LRR;
2. Table_array: the sample codes column in the SCR;
3. Col_index_num: 1;
4. [range_lookup]:FALSE (exact match)

15
December 2021
3
1. Check the sample codes in the LRR against the SCR.
Check whether all the sample codes in the LRR are included in the SCR. If some sample
codes included in the LRR are missing from the SCR, the DCFs may not have been
created yet in ADAMS for very recent tests within 21 days (the results are reported by the
laboratory before the DCFs are entered by the ADO), or there could be a mistake in the
TA field in the laboratory result created by the laboratory.
Corrective Actions for the ADOs:
Refer to your own files to verify if you had a testing mission at the date corresponding to
the collection dates of the missing sample codes. If so, verify your documentation to
ensure that all the DCFs were created in ADAMS. Refer to Section 5 of this Guide for
more information.
If the sample that is missing in the SCR is a double-blind sample for the laboratory quality
control purpose, you should remove this sample from the LRR.
If you have no mission corresponding to the collection dates of the missing codes, the
samples probably do not belong to your organization. Contact the laboratory to ask them
to verify the TA on the original copy of the corresponding DCFs or the chain of custody
and to modify the TA in the laboratory result in ADAMS accordingly.
In some cases, the Sample Collection Authority (if different from the TA) may need to be
contacted to provide clarification on the information included on the DCF original copy.
2. Check the sample codes in the SCR against the LRR.
Check whether all urine and blood sample codes in the SCR are also included in the LRR.
If some sample codes included in the SCR are missing from the LRR, the laboratory may
not have reported the results yet in ADAMS (normal pending results) or there could be a
mistake in the TA field in the laboratory result. The samples that are not found in the LRR
could have been reported under another ADO by the laboratory.
Corrective Actions for the ADOs:
Refer to your mission documentation to ensure the sample codes from the SCR are
correct. Refer to Section 5 of this Guide for more information. If it has been more than 20
working days since the sample were received by the laboratory (Article 5.3.8.4 of the
International Standards for Laboratories), contact the laboratory to follow up on the
pending results.
If the sample missing in the LRR is a sample that was not analyzed by the laboratory upon
agreement with the ADO, contact the laboratory and ask them to submit the result as “not
analyzed” in ADAMS.

16
December 2021
3
If all the information from the SCR is correct, contact the laboratory to ask them to verify
the TA on the original copy of the DCF and to modify the TA in the laboratory result in
ADAMS accordingly (if applicable).
3. Reject the match between a DCF and a laboratory result.
Once a DCF is matched to a laboratory result, certain fields in the laboratory result will
be locked from modification by the laboratory unless the match is rejected by the TA
and/or Results Management Authority (RMA). The process to reject a match is as
follows:
a) Search for the sample code in ADAMS and open the DCF page;
b) Find and click the yellow button of laboratory result (i.e. Negative Result, AAF or ATF)
at the bottom of the DCF;
c) Click on No-Reject Match on the Result Verification page.

17
December 2021
3
5. Monitor DCF Entry in ADAMS
1. Criteria to match a DCF and a laboratory result in ADAMS
In ADAMS, for a laboratory result (created by the laboratory) to be matched to its
corresponding sample’s DCF (created by the ADO), the information in the following five
fields must be identical:
Sample code
Sample type
Testing Authority
Sport
Laboratory
Only after the laboratory result and the DCF are matched will the TA be able to see the
laboratory result on the DCF page in ADAMS, as well as in the “Analysis Results”
column in the SCR.
2. Identify missing DCFs
After eliminating the data entry mistakes by following the instructions in Section 4 of this
Guide, generate a new LRR in ADAMS to create an updated Excel spreadsheet (follow
instructions of Section 2 of this Guide).
In this updated LRR, check the column “Testing Authority from DCF”. No data in this
column means that the laboratory result has not been matched to a DCF in ADAMS and
therefore the DCF for this sample is considered “missing”.

18
December 2021
3
3. Identify laboratory results not matched to existing DCFs
There is a possibility that the DCF has been entered in ADAMS but is not matched to the
corresponding laboratory result due to conflicting information in the five matching criteria.
Under such circumstance, the sample code is included in the SCR (based on DCF) but
shows no value in the “Testing Authority from DCF” column in LRR because of the no-
match.
It is also possible that the DCF is entered in ADAMS and that the information in the five
matching fields is also identical to the laboratory result, however there is still no value
displayed in the “Testing Authority from DCF” column of the LRR. This may be due to data
entry mistakes preventing the match between the laboratory result and the corresponding
DCF.
The following table shortlists have some common mistakes that can prevent the match
between an existing DCF and laboratory result for the same sample code in ADAMS. It is
important to carefully check the data for each sample code in question to identify the
reason of no-match and take the corrective action accordingly.

19
December 2021
3
Sample Code from
ADAMS DCF
(found on SCR)
Sample Code
from Lab
Result
(found on LRR)
Reason for No-match
Corrective Actions
01234 1234 “0” is considered a number at the beginning of
the sample code field.
Remove the “0” at the beginning of the sample
code field.
123O 1230 Letter “O” typed by mistake instead of number
“0” in the sample code field.
Correct letter “O” to number “0” in the sample
code field.
“01234 ” “1234 ” A space is considered a number at the
beginning or end of the sample code field.
Remove unnecessary spaces in the sample code
field.
1235
12388
1238 Any other mistakes in the sample code field
can prevent the match. Mistakes are often
due to misinterpretation of handwriting on the
original copy DCF or simply typos when the
ADAMS DCF was created; as the lab has
access to the sample bottle with official
sample code, their sample code is usually
correct.
Review the original copy DCF to confirm the
sample code number matches the lab’s and
correct the sample code field accordingly.
Boxing
Bowls
Para-Athletics
Sitting Volleyball
Table Tennis
Etc.
Kickboxing
Bowling
Athletics
Volleyball
Tennis
Etc.
Different sport codes. Review the original copy DCF to confirm the sport
of the athlete tested:
• If the sport field of the ADAMS DCF shows the
wrong sport, simply amend it accordingly.
• If the lab result shows the wrong sport, contact
the lab to ask that they amend the result
accordingly.
Blood passport
Blood
Different sample type.
Blood and blood passport are two different
sample types in ADAMS. If a whole blood
sample is analyzed for both ABP and another
prohibited substance(s), the sample code
needs to be entered twice as “blood” and
“blood passport” respectively on the DCF
page in ADAMS.
Review the original copy DCF to confirm the
sample type:
• If sample type field in the ADAMS DCF shows
the wrong sample type, simply amend it
accordingly.
• If the lab result shows the wrong sample type,
contact the lab to ask that they amend the
result accordingly.
Partial sample box is
checked
- ADAMS does not match a lab result to a
partial sample.
Uncheck the partial sample box in the ADAMS
DCF and save.
DCF status is
“Cancelled”
- ADAMS does not match a lab result to a
cancelled DCF.
• Use the "Correct" button* on top of DCF page
to delete the DCF;
• Enter a reason for deleting and save;
• Create a new DCF with the same sample code.
* If the “Correct” button does not appear on top of
your screen, contact the ADAMS Administrator of
your ADO to add this role to your ADAMS user
account.
Two zeros at the
beginning as part of the
sample code “0012123”
“__12123” ADAMS does not match a lab result to a DCF
that has a different sample code.
• Review the original copy DCF to confirm the
sample code number matches the lab’s and
correct the sample code field accordingly.
• Provide specific instructions to the lab as to
how report the leading zeros.

20
December 2021
3
6. TDSSA Monitoring
The new Next Generation (ADAMS NextGen) ADAMS Testing Center Modules include the
TDSSA Monitoring module. ADOs can use this tool to monitor their compliance with the TDSSA
requirements (i.e., the minimum levels of analysis (MLAs) for all sport/disciplines included in the
TDSSA) regularly. Once you have completed the tests and have entered DCFs into ADAMS, and
the results are reported by the laboratory, the TDSSA Monitoring module automatically calculates
your TDSSA implementation. If you identify any errors in DCF entry and correct them, it will be
automatically reflected in the TDSSA Monitoring module.
How to access ADAMS NextGen Testing Center and how to monitor your organization’s TDSSA
implementation can be found in ADAMS Help Center
.
Accessing Testing Center
TDSSA Monitoring
7. Contact
For further questions and inquiries regarding ADAMS account and reports, please contact the
ADAMS Support Team at adams@wada-ama.org
.
