
Effect of drying methods on the physical properties and microstructures
of mango (Philippine ‘Carabao’ var.) powder
O.A. Caparino
a
, J. Tang
a,
⇑
, C.I. Nindo
b
, S.S. Sablani
a
, J.R. Powers
c
, J.K. Fellman
d
a
Biological Systems Engineering Department, Washington State University, P.O. Box, Pullman, WA 99164-6120, USA
b
School of Food Science, University of Idaho, Moscow, ID 83844-2312, USA
c
School of Food Science, Washington State University, P.O. Box, Pullman, WA 99164-6120, USA
d
Horticulture and Landscape Architecture, Washington State University, P.O. Box, Pullman, WA 99164-6120, USA
article info
Article history:
Received 9 November 2011
Received in revised form 3 January 2012
Accepted 5 January 2012
Available online 4 February 2012
Keywords:
Drum drying
Freeze drying
Glass transition temperature
Microstructure
Physical properties
Refractance Window
Ò
drying
Spray drying
X-ray diffraction
abstract
Mango powders were obtained at water content below 0.05 kg water/kg dry solids using Refractance
Window
Ò
(RW) drying, freeze drying (FD), drum drying (DD), and spray drying (SD). The spray-dried
powder was produced with the aid of maltodextrin (DE = 10). The chosen drying methods provided wide
variations in residence time, from seconds (in SD) to over 30 h (in FD), and in product temperatures, from
20 °C (in FD) to 105 °C (in DD). The colors of RW-dried mango powder and reconstituted mango puree
were comparable to the freeze-dried products, but were significantly different from drum-dried (darker),
and spray-dried (lighter) counterparts. The bulk densities of drum and RW-dried mango powders were
higher than freeze-dried and spray-dried powders. There were no significant differences (P 6 0.05)
between RW and freeze-dried powders in terms of solubility and hygroscopicity. The glass transition
temperature of RW-, freeze-, drum- and spray-dried mango powders were not significantly different
(P 6 0.05). The dried powders exhibited amorphous structures as evidenced by the X-ray diffractograms.
The microstructure of RW-dried mango powder was smooth and flaky with uniform thickness. Particles
of freeze-dried mango powder were more porous compared to the other three products. Drum-dried
material exhibited irregular morphology with sharp edges, while spray-dried mango powder had a spher-
ical shape. The study concludes that RW drying can produce mango powder with quality comparable to
that obtained via freeze drying, and better than the drum and spray-dried mango powders.
Ó 2012 Elsevier Ltd. All rights reserved.
1. Introduction
Mango (Mangifera indica L.) is one of the most appreciated fruits
in the world. The 2005 world production of mango was estimated
at 28.5 million metric tons, of which 85% was produced in the fol-
lowing 10 countries: India (37.9%), China (12.9%), Thailand (6.3%),
Mexico (5.9%), Indonesia (5.2%), Pakistan (5.9%), Brazil (3.5%),
Philippines (3.5%), Nigeria (2.6%), and Egypt (1.3%) (Evans, 2008).
In the Philippines, mango ranks third among fruit crops next to
banana and pineapple in terms of export volume and value, with
a total of metric tons harvested in 2007. The Carabao variety pop-
ularly known as ‘‘Philippine Super Mango’’ accounts for 73% of the
country’s production (BAS, 2009). This variety is acclaimed as one
of the best in the world due to its sweetness and non-fibrous flesh.
Fresh mangoes are perishable and may deteriorate in a short
period of time if improperly handled, resulting in large physical
damage and quality loss, ranging from 5% to 87% (Serrano, 2005).
Gonzalez-Aguilar et al. (2007) reported that 100% of untreated ripe
mango fruits of the ‘Hadin’ variety showed fungal infection and se-
vere decay damage by the end of 18 days of storage at 25 °C. In or-
der to take advantage of the potential health benefits of mango and
add value to the commodity with lesser handling and transport
costs, there is a need to develop mango products in forms of mango
powders that not only have desired functionality but also are sta-
ble over a longer storage time. Mango powder offers several advan-
tages over other forms of processed mango products like puree,
juice and concentrate. Besides having a much longer shelf life
due to considerable reduction in water content, the transport cost
is also significantly reduced. Mango powders may also offer the
flexibility for innovative formulations and new markets. For exam-
ple, mango powders can be used as a convenient replacement for
juice concentrates or purees, and as shelf-stable ingredients for
health drinks, baby foods, sauces, marinades, confections, yogurt,
ice cream, nutrition bars, baked goods and cereals (Rajkumar
et al., 2007). Development of high quality mango powder may
match the increasing worldwide demand for more natural man-
go-flavored beverages either singly flavored or in multi-flavored
products (FAO, 2007), and meet the great demand for natural fruit
powders by the pharmaceutical and cosmetic industries.
0260-8774/$ - see front matter Ó 2012 Elsevier Ltd. All rights reserved.
doi:10.1016/j.jfoodeng.2012.01.010
⇑
Corresponding author. Address: 208 LJ Smith Hall, Pullman, WA 99164-6120,
USA. Tel.: +1 509 335 2140; fax: +1 509 335 2722.
Journal of Food Engineering 111 (2012) 135–148
Contents lists available at SciVerse ScienceDirect
Journal of Food Engineering
journal homepage: www.elsevier.com/locate/jfoodeng
Several drying technologies can be viable commercial options
for manufacture of mango powders, including freeze drying, drum
drying, spray drying and Refractance Window
Ò
drying. Each has its
own advantages and limitations. The final product obtained from
these methods may differ in physicochemical or nutritional prop-
erties and microstructures. Freeze drying, also known as lyophili-
zation, is a drying process in which the food is first frozen then
dried by direct sublimation of the ice under reduced pressure (Oet-
jen and Haseley, 2004; Barbosa-Cánovas, 1996). To carry out a suc-
cessful freeze drying operation, the pressure in the drying chamber
must be maintained at an absolute pressure of at least 620 Pa (To-
ledo, 2007). Freeze drying is generally considered as the best meth-
od for production of high quality dried products (Ratti, 2001). But,
it suffers from high production costs, high energy consumptions,
and low throughputs (Ratti, 2001; Hsu et al., 2003; Caparino,
2000).
Drum drying is commonly used in production of low moisture
baby foods and fruit powders (Kalogiannia et al., 2002; Moore,
2005). A drum dryer consists of two hollow cylinder drums rotat-
ing in opposite directions. The drums are heated with saturated
high temperature (120–170 °C) steam inside the drums. Raw mate-
rials are spread in thin layers on the outer drum surface and dry
rapidly. The product is scraped from the drum in the form of dried
flakes (Kalogiannia et al., 2002; Saravacos and Kostaropoulos,
2002). A major likely drawback is undesirable cooked aromas
and other severe quality losses in the final products caused by
the high temperature used in the drying process (Nindo and Tang,
2007).
Spray drying is widely used in commercial production of milk
powders, fruits and vegetables (Kim et al., 2009; Kha et al.,
2010). This method has several advantages, including rapid drying,
large throughput and continuous operation (Duffie and Marshall,
1953). During the drying process, the feed solution is sprayed in
droplets in a stream of hot air (Saravacos and Kostaropoulos,
2002). The liquid droplets are dried in seconds as a result of the
highly efficient heat and mass transfers (Toledo, 2007). The fin-
ished product can be made in the form of powder, granules or
agglomerates (Nindo and Tang, 2007). Spray drying processes can
be controlled to produce relatively free flowing and uniform spher-
ical particles with distinct particle size distribution (Barbosa-Cano-
vas et al., 2005; Duffie and Marshall, 1953). However, due to the
relatively high temperatures involved in spray-drying processes,
this drying technique may cause loses of certain quality and sen-
sory attributes, especially vitamin C, b-carotene, flavors and aroma
(Dziezak, 1988). In addition, it is difficult to directly spray dry su-
gar-rich materials such as mango, because they tend to stick to the
walls of the dryer (Bhandari et al., 1997a,b; Masters, 1985). Drying
aids, such as maltodextrin, are widely added to the feed to increase
glass transition temperature of the dried product and hence over-
come the problem of stickiness during spray drying.
Refractance Window
Ò
(RW™) is a novel drying technique de-
signed mainly to convert fruit puree into powder, flakes, or concen-
trates. The technology utilizes circulating hot water (95–97 °C) to
transfer thermal energy to a thinly spread liquid material placed
on a polyester conveyor belt that moves at a predetermined speed
while in direct contact with hot water. During drying, the thermal
energy from hot water is transmitted to foods through the plastic
conveyor by conduction and radiation. Water vapor from foods is
carried away by a flow of filtered air over the thin layer. This technol-
ogy offers several benefits when applied to fruits and vegetables. For
example, good retention of nutritional (vitamins), health-promoting
(antioxidants) and sensory (color, aroma) attributes were reported
for dried carrots, strawberries and squash (Nindo and Tang, 2007).
The bright green color of pureed asparagus remained virtually un-
changed when dried in the RW dryer, and was comparable to the
quality of freeze-dried product (Abonyi et al., 2002). In addition,
energy efficiency of RW drying method compares favorably with
other conventional dryers (Nindo and Tang, 2007).
Studies were reported that compared the influence of different
drying methods on various quality attributes of fruits and vegeta-
bles, including the color of dehydrated apple, banana, carrots and
potatoes (Krokida et al., 2001), b-carotene and ascorbic acid reten-
tion in carrots and strawberry (Abonyi et al., 2002), antioxidants
and color of yam flours (Hsu et al., 2003), asparagus (Nindo et al.,
2003), and antioxidant activities in soybean (Niamnuy et al.,
2011), encapsulated b-carotene (Desobry et al., 1997), and color
and antioxidant of beetroots (Figiel, 2010). However, no studies
have been conducted to evaluate the effect of drying methods on
mango powders in terms of color, bulk density, porosity, hygro-
scopicity, solubility, and microstructures. Thus, the objective of
this work was to investigate the influence of four drying methods
(Refractance Window
Ò
drying, freeze drying, drum drying and
spray drying) on the physical properties and microstructures of
resulting mango powders to provide better understanding in
selecting drying techniques that can be applied toward the manu-
facture of high quality mango powder.
2. Materials and methods
2.1. Preparation of mango puree
Frozen mango puree (Philippine ‘Carabao’ var.) was acquired
from Ramar Foods International (Pittsburg, CA). The puree was
produced following the manufacturer’s standard process that in-
volved selection of ripened mangoes (95–100% ripeness), washing
using chlorinated water, manual trimming, removal of any black
portions of the peel and separation of stone/peel. The cleaned man-
go fruits went through a pulping machine that separated the pulp
and discarded excess fibers. A buffer tank was used to standardize
the puree at 14–15 °Brix. The mango puree was pasteurized,
packed in 5 kg polyethylene (PE) bags, sealed and blast frozen at
35 °C. Bags of puree were placed in carton boxes and stored at
18 °C. The frozen mango puree was kept at constant temperature
while in transit from the Philippines to California and finally to
Washington State University (Pullman, WA). This frozen mango
was stored at 35 °C until it was ready for drying. The average
moisture content of the mango puree was 6.5 ± 0.1 kg water/kg
dry solids determined using standard oven method (AOAC, 1998).
2.2. Drying experiment
Frozen mango puree was thawed overnight at room tempera-
ture (23 °C), and afterward blended for 5 min to a uniform consis-
tency using a bench top blender (Oster Osterizer, Mexico) with
lowest speed setting. The puree was dried to below 0.05 kg
water/kg dry solids by Refractance Window
Ò
drying, freeze drying,
drum drying, or spray drying. Due to difficulty in spray drying of
this sugar-rich material, maltodextrin (DE = 10) (Grains Processing
Corporation, Muscatine, IA) was added to mango puree before
spray drying. No addition of carrier was used for the other three
drying systems. Detailed procedures for each drying method are
described below:
2.2.1. Refractance Window
Ò
drying
A pilot scale Refractance Window
Ò
dryer at MCD Technologies,
Inc. (Tacoma, WA) was used for drying mango puree. The dryer has
an effective surface drying area of 1.10 m
2
and length of 1.83 m in
the direction of belt movement. The main components of the dryer
included a conveyor belt made of ‘‘Mylar
Ò
’’ (polyethylene tere-
phthalate) plastic, a water pump, a hot water tank, a heating unit,
two water flumes, a hood with suction blowers and exhaust fans, a
136 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148

spreader, and a scraper (Fig. 1). The drying was accomplished by
spreading homogenized mango puree on the plastic conveyor belt
that moves over the surface of circulating hot water. The thickness
of the puree on the belt was 0.5–0.7 mm and was controlled using
a spreader bar. The thermal energy from the circulating hot water
(transferred to the puree through the belt) was used to remove
moisture from the product (Nindo et al., 2003). Previous studies re-
ported that the temperature of the product during drying rarely ex-
ceeded 80 °C(Abonyi et al., 2002). During drying operation, the
temperature of circulating hot water was maintained between 95
and 97 °C similar to that reported by Abonyi et al. (2002) and
Nindo and Tang (2007). The temperature of the circulating hot
water was continuously monitored at the flume inlet and outlet
section using pre-calibrated Type T thermocouple sensors. The sen-
sors were connected to a data acquisition unit equipped with mon-
itoring software. Water vapor removal from the samples was
facilitated by forcing the suction air (22 °C) with relative humidity
(50–52%) over the puree at an average air velocity of 0.7 m/s (Abo-
nyi et al., 2002). The residence time to dry the mango puree into
flakes or powder was determined by monitoring the time travelled
by the thinly spread mango puree from inlet to the outlet section of
the plastic conveyor belt. Measurement of the residence time was
performed in triplicate.
2.2.2. Freeze drying
Freeze drying was carried out using a laboratory freeze dryer
(Freeze Mobile 24, Virtis Company, Inc., Gardiner, NY). The thawed
mango puree was poured into a stainless pan to form a layer of
15 mm. The samples were placed at 25 °C for 24 h before trans-
ferring to the freeze dryer. The vacuum pressure of the dryer was
set at 20 Pa, the plate temperature was 20 °C, and the condenser
was at 60 °C. The residence time needed to dry the mango puree
to below 0.05 kg water/kg dry solids was determined when the
vacuum pressure had dropped to 30 mTorr (4 Pa).
2.2.3. Drum drying
A laboratory atmospheric double drum dryer (Model no. ALC-5,
Blaw-Knox Co., Buffalo, NY) was utilized in this experiment. The
dryer has two hollow metal drums with 0.15 m external diameter
and 0.19 m length. The drums were internally heated by steam at
379.2 ± 7 kPa producing a temperature of 152 ± 2 °C. Preliminary
experiments were conducted at different rotational speed settings
in order to obtain dried sheets of below 0.05 kg water/kg dry solids.
The clearance between the two drums was fixed at 0.01 mm allow-
ing the puree to flow (forced by rotary action) into a thin layer as it
passed through the gap. The drum temperature was allowed to
stabilize before feeding the puree. This prepared puree was poured
evenly over the hot pool area between the two drums. After trav-
eling approximately three fourths of the revolution of the drums
or 15 cm distance, the dried product was scraped from the drum
surface by doctor blades. The residence time for drying was re-
corded by taking three fourths of the time measured for one com-
plete revolution of the drum. Due to stickiness of mango, the dried
product at the exit section of the dryer tended to roll and build up
while the drum was rotating forming an extruded-like product and
not the expected thin flakes. Thin sheet or flakes of dried product
was obtained by carefully pulling the dried product as it goes out
of the exit section of the dryer. The dried product removed from
the two drums was mixed together for analysis because their
appearance and moisture content were generally similar.
2.2.4. Spray drying
The thawed mango puree was spray-dried in a pilot scale S-1
spray dryer (Anhydro Attleboro Falls, MA). Before starting the
experiment, the dryer was conditioned for 20 min by pumping
de-ionized water through the atomizer with the dryer inlet and
outlet temperatures set at 180 and 80 °C, respectively (Shrestha
et al., 2007). The mango puree was pumped into the spray dryer
chamber at a flow rate of 50 ± 2 g/min using Masterflex pump
(Cole-Parmer Instruments Co., Chicago, IL). The air temperature
was maintained at 190 ± 2 °
C (dryer inlet) and 90 ± 2 °C (dryer out-
let) during drying. These air inlet and outlet conditions are within
the recommended temperatures of 180–220 and 90–110 °C,
respectively, for spray drying of heat sensitive products at atmo-
spheric pressure (Filkova and Mujumdar, 1995; Kim et al., 2009).
The outlet temperature determines the thermal exposure of the
sample during spray drying. It was observed during preliminary
experiments that spray drying of mango puree without any carrier
was not possible due to the high content of low molecular weight
sugars (e.g. fructose, glucose, sucrose), similar to what had been re-
ported by other authors (Abonyi et al., 2002; Bhandari et al.,
1997a,b). Maltodextrin (DE = 10) having a median glass transition
temperature of T
gm
= 139.7 °C(Jakubczyk et al., 2010) was added
to produce a non-sticky and free flowing powder (Bhandari et al.,
1997a,b). Preliminary experiments were carried out to obtain dried
product that has better appearance and throughput. Three malto-
dextrin concentrations of 0.25, 0.35 and 0.45 kg/kg dried mango
solids were tested for this purpose (Jaya et al., 2006; Nindo and
Tang, 2007; Sablani et al., 2008). By visual examination, the color
and appearance of the dried mango powder from the three treat-
ments showed very little variation. Hence, the spray-dried mango
powder with the lowest maltodextrin concentration of 0.25 kg/kg
dried mango solids was selected for comparison with other dried
powders. The actual residence time to obtain mango powder with
Fig. 1. Schematic layout of Refractance Window
Ò
dryer (adapted from Nindo and Tang (2007) and Abonyi et al. (2002)).
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
137

a moisture content below 0.05 kg water/kg dry solids was not mea-
sured, but the information from previous studies on spray drying of
sugar-rich material was used to approximate the time.
2.3. Handling and packaging of samples
The product from each drying process had unique geometries at
the exit point, so different handling procedures were employed.
Rectangular cake-like dried products obtained from the freeze dry-
ing process were collected and sliced into smaller pieces using a
clean stainless steel knife and packed in leak-proof Ziploc
Ò
plastic
bags. The spray-dried material, which appeared like agglomerated
spherical shapes, was immediately packed in the same type of
plastic bags after coming out from the dryer. The dried thin sheets
collected from the drum and RW drying processes were handled in
a similar manner. All the samples sealed in Ziploc
Ò
bags were
placed inside aluminum-coated polyethylene bags. To prevent oxi-
dation, all the packaged samples were flushed with nitrogen gas,
heat sealed and stored at 35 °C until further analyses.
2.4. Grinding and sieving
One hundred grams each of dried flakes or sheets obtained from
different drying processes were ground using mortar and pestle.
Sieving analysis was carried out by stacking and vibrating the
sieves in ascending order of mesh sizes of 35, 45, 60 and 80 (Amer-
ican Society for Testing and Materials, ASTM) to obtain particle
sizes of 500, 350, 250 and 180
l
m (International Standard for
Organization, ISO), respectively (Barbosa-Canovas et al., 2005).
Those with particle sizes ranging between 180–500
l
m and flakes
or sheets were evaluated in terms of color, bulk density and bulk
porosity, while particle sizes of 180–250
l
m were analyzed for sol-
ubility, hygroscopicity and microstructures.
2.5. Water content
The water content of mango puree and dried flakes/powders
made from RW, freeze, drum and spray drying methods was deter-
mined using the standard oven method at 70 °C and 13.3 kPa for
24 h (AOAC, 1998). The drying, cooling and weighing of samples
was continued until the difference between two successive weigh-
ing was less than 1 mg.
2.6. Water activity
Water activity of the RW-, freeze-, drum-, and spray-dried man-
go powders was measured using water activity meter (Aqualab 3TE
series, Decagon Devises, Pullman, WA). Duplicate samples were
measured at 24.7 ± 1 °C.
2.7. Physical properties of mango powders
2.7.1. Color analysis
The dried mango in flakes or sheet forms and four different par-
ticle sizes of 500, 350, 250 and 180
l
m were evaluated for color
comparison. Mango powders or flakes were poured into Petri
dishes, slightly shaken to form a layer of 10 mm thickness and cov-
ered with transparent film (Saran™ Wrap, SC Johnson, Racine, WI).
The International Commission on Illumination (CIE) parameters L,
a
⁄
and b
⁄
were measured with a Minolta Chroma CR-200 color me-
ter (Minolta Co., Osaka, Japan). The colorimeter was calibrated with
a standard white ceramic plate (L = 95.97, a = 0.13, b = 0.30)
prior to reading. Corresponding L value (lightness of color from
zero (black) to 100 (white); a
⁄
value (degree of redness (0–60) or
greenness (0 to 60); and b
⁄
values (yellowness (0–60) or blueness
(0 to 60) were measured for all the samples. The average L, a
⁄
and
b
⁄
values were obtained from six readings taken from each of five
locations. The hue angle, H
⁄
and chroma, C
⁄
expressed as H
¼
tan
1
b
a
and C
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
a
2
þ b
2
p
, respectively were also calculated (Abo-
nyi et al., 2002). Hue is a color attribute by which red, yellow, green
and blue are identified, while chroma distinguishes between vivid
and dull colors.
For color comparison with the original mango puree, 2 g each of
RW-, freeze-, drum-, and spray-dried mango powders (250
l
m)
with water content of 0.017 ± 0.001, 0.023 ± 0.002, 0.013 ± 0.001
and 0.043 ± 0.003 kg water/kg dry solids were reconstituted by
adding an amount of 12.10, 12.04, 11.96 and 11.70 g of distilled
water, respectively using material balance. The reconstituted man-
go powders produced slurries with moisture content of 6.143 kg
water/kg dry solids similar as the original mango puree. The recon-
stitution of mango powder was carried out by mixing the powder
and water at 23 °C while vortexing (Fisher Scientific mini vortexer,
USA) until the powder was completely dissolved. The L
⁄
, a
⁄
and b
⁄
,
H
⁄
and C
⁄
values were immediately measured and calculated fol-
lowing the same procedure employed for mango flakes and pow-
ders. The total change in color of the reconstituted mango
powders with reference to the original puree were computed as:
D
E ¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
ðL
0
L
Þ
2
þða
0
a
Þ
2
þðb
0
b
Þ
2
q
where, subscript ‘‘o’’ de-
notes the color of original puree (Jaya and Das, 2004; Nindo
et al., 2003).
2.7.2. Bulk density
The bulk density of the mango powder obtained from different
drying processes and particle sizes was measured following the
procedure described in previous studies with modification (Barb-
osa-Canovas et al., 2005; Goula and Adamopoulus, 2008). Approx-
imately 5 g of mango powder was freely poured into a 25 ml glass
graduated cylinder (readable at 1 ml) and the samples were
repeatedly tapped manually by lifting and dropping the cylinder
under its own weight at a vertical distance of 14 ± 2 mm high until
negligible difference in volume between succeeding measure-
ments was observed. Given the mass m and the apparent (tapped)
volume V of the powder, the powder bulk density was computed as
m/V (kg/m
3
). The measurements were carried out at room temper-
ature in three replicates for all samples.
2.7.3. Particle density and bulk porosity
The particle densities of mango powders obtained by different
drying methods were calculated by adopting the pycnometer
method. A 2.5 ± 0.04 g of each of the RW-, freeze-, drum-, and
spray-dried mango powders (180–250
l
m) was placed in an
empty liquid pycnometer (25 ml), and filled with measured vol-
ume of toluene. Toluene was used because of its ability to pene-
trate the finest external pores connected to surface of the
material without dissolving the material. Bulk porosity (
e
b
) was
calculated by determining the ratio of particle density (
q
p
) and
bulk density (
q
b
) using the Eqs. (1)–(3) as (Krokida and Maroulis,
1997):
q
b
¼
m
s
V
t
ð1Þ
q
p
¼
m
s
V
s
ð2Þ
e
b
¼ 1
q
b
q
p
ð3Þ
where
q
b
is the bulk density of mango solids,
q
p
is the particle den-
sity of the solids, m
s
is the mass of mango solids, V
t
and V
s
is the to-
tal and volume of the dry solids, respectively.
138 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148

2.7.4. Solubility
Solubility of mango powder was determined using the procedure
developed by Eastman and Moore (1984) as adopted by Cano-Chau-
ca et al. (2005). One gram of the powder (dry basis) was dispersed in
100 ml distilled water by blending at high speed (13,000 rpm) for
5 min using an Osterizer blender (Oster, Mexico). The dispersed
mango powder was then centrifuged at 3000g for 5 min. A 25 ml ali-
quot of the supernatant was carefully pipetted and transferred to a
pre-weighed aluminum dish and then oven-dried at 105 °C for 5 h.
Drying was continued and weighed every hour for 2 h. The solubility
of the powder (%) was determined by taking the weight difference.
2.7.5. Hygroscopicity
Ten grams each of RW-, freeze-, drum- and spray-dried mango
powders with particle sizes of 180–250
l
m and moisture content
below 0.05 kg H
2
O/kg mango solids were placed in an open glass
container. Three replicate samples for each product were put sep-
arately in three sealed humidity jars containing NaCl saturated
solution (75.5% humidity) and stored at 25 °C for 7 days. Samples
were prepared at 20 °C. Hygroscopicity, HG (%) or 1 g of adsorbed
moisture per 100 g dry solids (g/100 g) was calculated using the
following equation:
HG ¼
D
m=ðM þ M
i
Þ
1 þ
D
m=M
ð4Þ
where
D
m (g) is the increase in weight of powder after equilibrium,
M is the initial mass of powder and M
i
(% wb) is the free water con-
tents of the powder before exposing to the humid air environment
(Jaya and Das, 2004; Sablani et al., 2008; Tonon et al., 2008).
2.8. Glass transition temperature
Glass transition temperature (T
g
) of mango powders with water
activity below 0.2 was measured using differential scanning
calorimeter (DSC, Q2000, TA Instruments, New Castle, DE), follow-
ing the procedure described by Syamaladevi et al. (2009). The
calorimeter was calibrated for heat flow and temperature using
standard indium and sapphire. Twelve to sixteen milligrams of
each mango powder sample was sealed in an aluminum pan
(volume of 30
l
l), cooled down from 25 to 90 °C using liquid
nitrogen and then equilibrated for 10 min. The samples at 90 °C
were scanned to 90 °C then cooled down to 25 °C. Scanning of all
samples was carried out using the same heating and cooling rate
of 5 °C/min. To avoid condensation on the surface of the powder
particles, a nitrogen carrier gas was purged at a flow rate of
50 ml/min. The onset- (T
gi
), mid- (T
gm
) and end-point (T
ge
) values
of the mango powders were determined by finding the vertical
shift in the heat flow-temperature diagram. All measurements
were performed in duplicate.
2.9. X-ray diffraction
X-ray diffraction (XRD) characteristics of mango powders
obtained from different drying processes were investigated using
a Siemens D-500 diffractometer (Bruker, Karlsruhe, Germany).
The powder samples (180–250
l
m) were placed and slightly
pressed in an aluminum holder using a glass slide. The diffractom-
eter was operated at a wavelength of 0.15 nm and the input energy
was set at 30 mA and 35 kV. Diffractograms were taken between 5°
and 50° (2h) with a step angle of 0.02° and scan rate of 1 s per step.
The XRD patterns of all the samples were plotted for comparison.
2.10. Microstructure analyses
A small quantity of mango powders (180–250
l
m) from differ-
ent drying systems were mounted on aluminum stubs and coated
with a fine layer of gold (15 nm) using a Sputter gold coater (Tech-
nics Hummer V, Anatech, San José, CA). All powder samples were
examined by Scanning Electron Microscopy using SEM Hitachi S-
570 camera (Hitachi Ltd., Tokyo, Japan) operated at an accelerating
voltage of 20 kV. Micrographs were photographed at a magnifica-
tion of 100, 300 and 1000 at scale bar of 0.30 mm, 100
l
m
and 30
l
m.
The microstructure of samples prepared for hygroscopicity
experiments were also analyzed to identify possible relationships
between the obtained hygroscopicity values for each mango pow-
der sample using a Quanta 200F Environmental Scanning Electron
Microscope (FEI, Field Emission Instruments, Hillsboro, Oregon,
USA). The low vacuum mode (200 Pa) was used during scanning
to allow measurement of samples at their native state. Observa-
tions were carried out with an accelerated voltage of 30 kV and
magnification of 700 at a scale of 100
l
m.
2.11. Statistical analysis
All experiments were carried out at least in duplicate, the re-
sults analyzed using the general linear model procedure of SAS
(SAS Institute Inc., Cary, NC), and the means separated by Tukey-
honest significant difference test with a confidence interval of
95% used to compare the means. Mean standard deviations are pre-
sented in the results.
3. Results and discussion
3.1. Residence time, water content and product temperature
The residence time during drying of mango puree from the ini-
tial moisture content of 6.52 kg water/kg mango solids to below
0.05 kg water/kg mango solids was accomplished in 180 ± 0.15,
111,600 ± 5100 and 54 ± 0.2 s for RW, FD and FD, respectively,
and less than 3 s with SD (Table 1). It should be noted here that
the residence time used for SD was only an approximation based
on the data reported by Desobry et al. (1997) and Jayasundera
et al. (2011b). The actual residence time during spray drying of
mango powder in our study might be higher than 3 s because of
the difference in drying conditions and specifications of the spray
dryer used as compared from the literature. Nevertheless, the esti-
mated residence time for SD is definitely much smaller than for
RW, freeze and drum drying. The product temperatures measured
for each drying process was 74 ± 2 °C (RW), 20 ± 0.5 °C (FD),
105 ± 5 °C (DD) and 90 ± 2 °C (SD).
3.2. Physical properties of mango powder
3.2.1. Color analysis
The color of the dried product (mango flakes/sheet) or powders
of different particle sizes were affected by the drying methods.
Table 1
Drying conditions for production of mango powders using different methods.
Product Product
temperature (°C)
Residence
time (s)
Water content
(kg water/kg dry solids)
Fresh puree – – 6.518 ± 0.123
RW 74 ± 2 180 ± 0.15 0.017 ± 0.001
FD 20 ± 0.5 111,600 ± 5091 0.023 ± 0.002
DD 105 ± 5 54 ± 0.2 0.013 ± 0.001
SD 90 ± 2 1–3
a
0.043 ± 0.003
Standard deviation from the average value of at least two replicates.
a
The residence time was an approximate value, based on information given in
Desobry et al. (1997) and Jayasundera et al. (2011a,b,c).
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
139

Visual examination showed that spray-dried (agglomerate powder
particles) and drum-dried mango powder had the lightest and
darkest color, respectively. The color difference between mango
powder obtained using RW and FD was not significantly different
(P 6 0.05) (Fig. 2). Hunter color tristimulus values for mango pow-
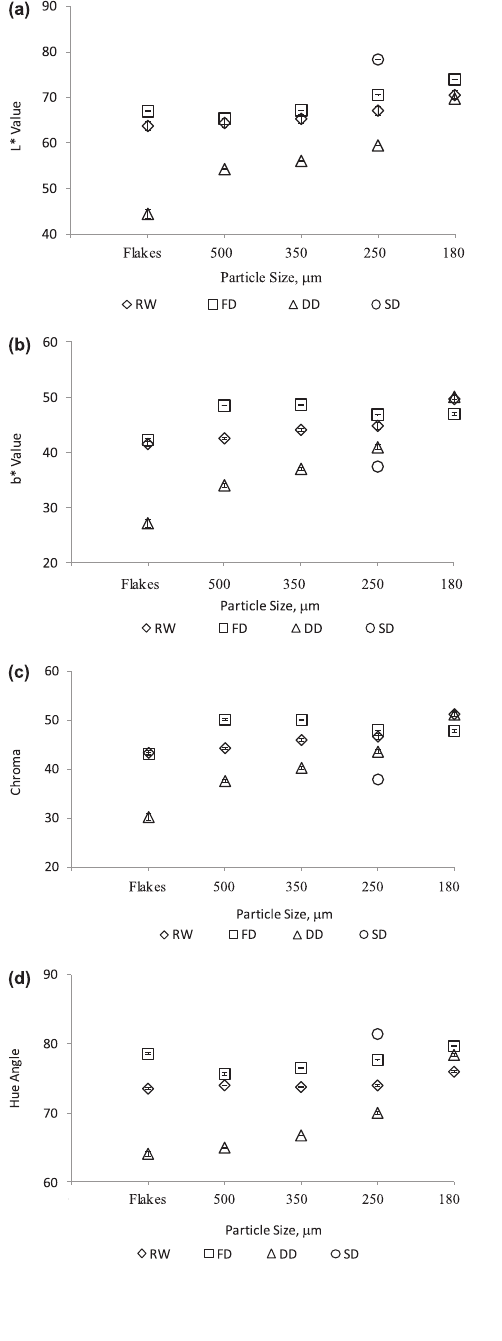
der of different particle sizes are presented in Fig. 3. Overall, the
product (flakes) at exit had a significant difference in the L value
(lightness) among the RW-, freeze-, drum-, and spray-dried mango
flakes or powders, except for the RW and freeze-dried powder with
particle size of 500 and 350
l
m which showed no significant vari-
ation (P 6 0.05) (Fig. 3a). The similarity in L-value for RW, FD and
DD powders of the smallest particle size (180
l
m) may be attrib-
uted to negligible effect on reflectance.
The mango powder produced by spray drying had the highest L
value, while the drum-dried mango powder appeared to have the
lowest L value (indicating darkest color). The lighter color in spray
drying was due to the addition of maltodextrin carrier which was
necessary to reduce the stickiness of the mango to allow the spray
drying process to be effective (Abonyi et al., 2002; Jaya et al., 2006).
While the outlet temperature during spray drying reached
90 ± 2 °C, the drying time was very short (l–3 s) as reported by
Desobry et al. (1997). Hence the color degradation was limited.
On the other hand, the darker color of the drum-dried mango
powder can be attributed to high drying temperature. Such effect
confirmed previous studies on strawberry puree (Abonyi et al.,
2002) wherein color degradation was greatly influenced by high
processing temperatures. The dark color in drum-dried mango
flakes or powder can be characterized by browning reaction or
Maillard reaction caused by the chemical reactions between sugars
and proteins (Potter and Hotchkiss, 1995). Moreover, carameliza-
tion of sugars in mango can occur due to high temperature contrib-
uting to darkening during drying. The dominant color in mango
puree is yellow and hence can be best represented by Hunter color
b
⁄
(yellowness) to distinguish the color difference of the resulting
mango powders as affected by the drying process. No significant
difference was observed in b
⁄
value (yellowness) between RW
and freeze-dried mango powder while there was a highly signifi-
cant difference between spray and drum-dried product (P 6 0.05)
(Fig. 3b). Chroma value or vividness in yellow color of 250
l
m
particle size RW and freeze dried mango flakes and powders
showed no significant difference, but RW-dried mango powder
with particle size 350–500
l
m were of a more vivid yellow color
than freeze dried mango powder having obtained the highest chro-
ma value (Fig. 3c). The hue angle value in spray-dried mango pow-
der was the highest but its chroma value is very low indicating a
dull color (Fig. 3d). RW dried mango flakes or powder at all particle
sizes obtained a higher hue angle compared to freeze and drum-
dried mango powders suggesting that RW-dried mango powder is
more vivid in its yellow color implying that it will be more attrac-
tive and appealing to consumers. The overall distinct vivid yellow
color of the RW-dried mango may be indicative of high b-carotene
retention. Abonyi et al. (2002) reported that b-carotene in RW and
freeze-dried carrot puree was 53% and 55% higher compared to
drum-dried products, respectively. Wagner and Warthesen (1995)
reported that the yellow and red color of carrot slices is attributed
to the presence of carotenes. Also, the b
⁄
(yellow) values for raw and
puree sweet potato were highly correlated with b-carotene content
(Ameny and Wilson, 1997). The minimal color change of product
produced by RW and freeze drying suggests the appropriateness
of these processes to produce high quality products. The compara-
ble yellow color of RW and freeze-dried mango powder can also be
attributed to low product temperature for RW (74 ± 2 °C) and
freeze-dried (20 ± 0.5 °C), compared to spray-dried (90 ± 2 °C) and
drum-dried (105 ± 5 °C) mango powder.
The reconstituted mango powder was prepared by adding water
to achieve the same solid contents as the original mango puree.
Visual examination of the color of the reconstituted RW-, freeze-,
drum-, and spray-dried mango powders showed variations in com-
parison with the original mango puree (Fig. 4). Luminosity (L
⁄
) val-
ues as presented in Table 2 showed no significant difference
between reconstituted RW- and freeze-dried mango puree and
both are similar in luminosity to the original puree. Reconstituted
drum-dried mango puree was darker as expected because of the
darker powder. The result is in agreement with the work of Abonyi
et al. (2002) wherein a drum-dried carrot puree was perceived as
darker in comparison with powders produced by spray, freeze
Fig. 2. Photograph of mango flakes or powders at different particle sizes obtained from Refractance Window
Ò
(RW) drying, freeze drying (FD), drum drying (DD), and spray
drying (SD).
140 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
and RW drying methods. Spray-dried mango powder was darker
than RW- and freeze-dried but lighter than reconstituted drum
dried mango puree. The original mango puree and reconstituted
RW and freeze-dried mango powders are significantly different
but comparable in terms of vividness and saturation of yellow
color. On the other hand, the reconstituted drum-dried and
spray-dried mango puree had lower chroma values indicating less
saturation and dull yellow appearance. A comparable result was
also observed for hue angle among the original puree, reconstituted
RW- and freeze-dried mango puree while reconstituted drum-dried
mango puree had a low hue angle value, which indicates a dull
yellow color. The reconstituted spray-dried mango puree had the
highest hue angle value but because of its low lightness and chroma
values, it produced a grayish pale color. The reconstituted mango
powder from drum drying process showed the highest deviation
in color with respect to the original mango puree having a
D
E value
of 9.22 ± 0.01 followed by the reconstituted spray-dried mango
puree with
D
E value of 6.23 ± 0.02 (e.g. lightest). The reconstituted
RW-dried mango puree had the lowest color difference with
D
E va-
lue = 1.22 ± 0.02, a value very close to reconstituted freeze-dried
mango puree with
D
E value = 1.57 ± 0.02. The distinct superiority
of RW drying process against drum and spray drying processes in
producing mango powder in the present experiment is in corrobo-
ration with previous studies for asparagus (Nindo et al., 2003), and
carrots and strawberry (Abonyi et al., 2002).
3.2.2. Bulk density and porosity
For all drying methods, the bulk density of mango powders in-
creased and their porosity decreased with decreasing particle size
(Figs. 5 and 6). These results may be attributed to the decrease in
the inter-particle voids of smaller sized particles with larger
contact surface areas per unit volume. Similar observation was re-
ported for bulk density of ginger powder at different particle sizes
(Xiaoyan, 2008). It was also consistent with the explanation by
other authors that powder characteristics such as particle size
may result in significant changes in bulk density and porosity
(Barbosa-Canovas et al., 2005).
Freeze- and spray-dried mango powders had significantly lower
bulk densities and higher porosities compared to drum- and RW-
dried products (P 6 0.05) (Figs 5 and 6). It is well recognized that
in freeze drying of foods in the form of either puree or as a whole,
the material is first frozen allowing it to maintain its structure fol-
lowing sublimation of ice under high vacuum (Oetjen and Haseley,
2004). Since liquid phase in the material is not present during this
process, there is no transfer of liquid water to the surface, but in-
stead the ice changes to vapor below the collapse temperature
without passing the liquid state (Krokida and Maroulis, 1997). In
effect the collapse and shrinkage of the product is prevented there-
by resulting in a porous dried material (Karel, 1975).
The higher porosity or lower bulk density in spray-dried mango
powder was due to the addition of maltodextrin (Fig. 6). Shrestha
et al. (2007) demonstrated that increasing maltodextrin concentra-
tion in tomato pulps led to the decrease in bulk density. Goula and
Adamopoulus (2008) also explained that maltodextrin is consid-
ered a skin-forming material and by using it as carrier can induce
accumulation and trapping of air inside the particle causing it to
become less dense and porous.
On the other hand, the bulk porosity and density of RW- and
drum-dried mango powder were significantly lower and higher
than freeze and spray dried product, respectively with drum dried
product exhibited the lowest porosity (P 6 0.05) (Figs. 5 and 6).
During drum drying, the mango puree poured inside a pool
between the two drums has vapor bubbles bursting at the free sur-
face and spattered along side of the two drum surfaces as triggered
by high temperature (above boiling). The high temperature used in
Fig. 3. Lightness (a), yellowness (b), chroma (c) and hue angle (d) of mango flakes
or powders at different particle sizes obtained from Refractance Window
Ò
(RW),
freeze drying (FD), drum drying (DD), and spray drying (SD).
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
141

drum drying may have caused collapse which resulted in more
compact and rigid product. These characteristics resulted in lower
porosity when compared to freeze- or spray-dried mango powder.
RW-dried mango powder exhibited low porosity compared to
freeze- and spray-dried mango powder but significantly higher
than drum-dried powder (P 6 0.05) (Figs. 5 and 6). RW is catego-
rized as a direct drying technique similar to drum-drying (Nindo
and Tang, 2007), except that the energy is indirectly transferred
via plastic film instead of steel as in drum drying. Apparently, both
drying processes seem to produce a similar form of end product.
3.2.3. Solubility
Solubility is the most reliable criterion to evaluate the behavior
of powder in aqueous solution. This parameter is attained after the
powder undergoes dissolution steps of sinkability, dispersability
and wettability (Chen and Patel, 2008). There was no significant
difference in the solubility between spray and drum-dried mango
powder, while both were significantly higher compared to RW
and freeze-dried product (P 6 0.05) (Table 3). The high solubility
of spray-dried mango powder can be attributed to the addition of
maltodextrin (DE = 10). This result was in agreement with the
study reported by Cano-Chauca et al. (2005) where they concluded
that solubility of mango powders increased when maltodextrin
was added during spray-drying. Maltodextrin is a material that
serves as coating agent as the particle crust is developed during
spray drying resulting in a product that is highly soluble (Desai
and Park, 2004). Cai and Corke (2000) also confirmed that malto-
dextrin as a carrier and coating agent increased the solubility of
spray-dried betacyanins. The atomization of mango puree during
spray drying may also contribute to solubility of spray-dried prod-
uct. Fibers present in mango might have been broken into tiny
pieces as a result of high atomization of the material resulting in
increased solubility. From the above observations, maltodextrin
was proven effective in increasing solubility of spray-dried mango
powder. However, spray drying of mango puree containing
25 kg/kg dried mango solids significantly altered the total color
change of the resulting mango powder as earlier discussed. Like-
wise, the cyclone recovery of mango powder at this maltodextrin
concentration was only 37.8 ± 1.8% (data not shown), far below
the >50% benchmark cyclone recovery for a marginally successful
spray drying process of sugar-rich material (Bhandari et al.,
Fig. 4. Photograph of reconstituted mango powders obtained from Refractance Window
Ò
(RW), freeze drying (FD), drum drying (DD), and spray drying (SD).
Table 2
Hunter color measurements of reconstituted mango powders obtained from different drying processes.
Drying method L
⁄
a
⁄
b
⁄
C
⁄
Hue angle b
⁄
/a
⁄
D
E
Original puree 45.12 ± 0.02
a
4.65 ± 0.01
c
41.52 ± 0.03
a
41.78 ± 0.03
c
83.61 ± 0.01
a
8.93 ± 0.01
c
–
RW 43.95 ± 0.02
b
4.40 ± 0.01
d
41.79 ± 0.03
a
42.02 ± 0.03
b
83.99 ± 0.01
a
9.50 ± 0.02
b
1.22 ± 0.02
d
FD 43.74 ± 0.06
c
4.69 ± 0.01
b
40.99 ± 0.23
b
41.26 ± 0.23
d
83.47 ± 0.04
b
8.73 ± 0.06
d
1.57 ± 0.03
c
DD 37.73 ± 0.01
d
6.92 ± 0.02
a
36.48 ± 0.02
c
37.13 ± 0.02
e
79.27 ± 0.03
c
5.28 ± 0.03
e
9.22 ± 0.01
a
SD 41.59 ± 0.07
e
3.05 ± 0.01
e
36.64 ± 0.02
c
36.77 ± 0.03
a
85.24 ± 0.02
d
12.00 ± 0.05
a
6.23 ± 0.02
b
D
E is calculated using the original mango puree as reference.
a–e
Means with the same superscript letters within a column indicate no significant differences (P 6 0.05).
0
200
400
600
800
1000
RW FD DD SD
Bulk Density,k g/m3
Drying method
500 350 250 180
µm µm µm µm
Fig. 5. Bulk density of mango powders obtained from Refractance Window
Ò
(RW)
drying, freeze drying (FD), drum drying (DD), and spray drying (SD).
0.00
0.10
0.20
0.30
0.40
0.50
0.60
RW FD DD SD
Porosity
Drying method
500 350 250 180
µm µm µm µm
Fig. 6. Porosity of mango powders obtained from Refractance Window
Ò
(RW)
drying, freeze drying (FD), drum drying (DD), and spray drying (SD).
142 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148

1997a,b; Jayasundera et al., 2011a,b). The application of alternative
drying aids such as proteins and low molecular surfactants may
improve the recovery and quality of spray-dried mango powders
and help in maintaining higher solubility (Jayasundera et al.,
2011a,b,c; Adhikari et al., 2009a,b). Recently, a type of protein
called ‘‘Protein X’’ developed at the University of Sydney, was
found to increase the recovery of sugar-rich material of up to
80% by just adding a small amount (<5%) to the sticky fruit juice
or puree (Wang et al., 2011).
Drum dried mango powder also had a high solubility value that
was not significantly different from spray-dried mango powder.
The higher solubility of drum-dried samples could be attributed
to a higher degree of macromolecular disorganization of the mate-
rial as affected by drying process and condition. However, its infe-
rior dark color may restrict consumer acceptance even though its
solubility is high.
The solubilities of freeze- and RW-dried mango powders were
similar and significantly lower than that of spray- and drum-dried
mango powders. Both drying methods are gentle in terms of prod-
uct temperature (Table 1). One possible reason for the lower solu-
bility of those samples is that the cell structure of mango puree
was not disrupted and smaller amounts of solids were dissolved
to become part of the supernatant.
3.2.4. Hygroscopicity
A demarcation or cut-off values for hygroscopicity (HG) of man-
go powder ranging from 5.13% to 9.38% were considered as the ba-
sis for comparing the results in our study. These figures were based
on the average range of hygroscopicity values of instant coffee
(lower HG) and tomato soup powder (higher HG) as calculated
by Jaya and Das (2004). Table 3 shows the hygroscopicity of mango
powders made from Refractance Window, freeze, drum and spray
drying. The drum-dried mango powder exhibited the highest
hygroscopicity (20.1 ± 0.88%), 74% higher than the higher limit
cut-off HG, indicating its strong capacity to attract water molecules
when in contact with the surrounding air. Mujumdar (2007) ex-
plained that drum drying of sugar-rich fruits requires high temper-
ature and usually a dry to very low moisture thin sheet product.
This drying condition usually causes the product to be very hygro-
scopic and often other quality attributes are degraded. The lower
hygroscopicity value obtained for spray drying (16.5 ± 0.06) or
68.91% higher than the benchmark HG can be attributed to the
addition of maltodextrin in the mango puree before drying. Tonon
et al. (2008) demonstrated that the hygroscopicity of spray-dried
acai powder gets lower as the concentration of maltodextrin was
increased. Similar observation was confirmed during spray drying
of cactus pear juice (Rodríguez-Hernández et al., 2005), sweet po-
tato powder (Ahmed et al., 2009) and betacyanin pigments (Cai
and Corke, 2000), suggesting that maltodextrin is an efficient car-
rier agent in lowering hygroscopicity of dried material. Conse-
quently, when this carrier is added, other quality attributes of
the mango powder such as color and b-carotene (data not shown)
were found inferior. There was no significant difference in the
hygroscopicity between RW- (18.0 ± 0.36%) and freeze-dried
(18.0 ± 0.19%) mango powder (P 6 0.05) obtaining a similar in-
crease of 71.50% based on the higher limit cut-off HG, suggesting
the superiority of RW over drum and spray-dried mango powder.
The small variation of moisture content of the different samples
may have direct relationship with the hygroscopicity as shown in
Table 3. Tonon et al. (2008) expound that the low moisture
spray-dried acai has the greater capacity to absorb water from
the surrounding air and hence is more hygroscopic. However, his
findings on the moisture–hygroscopicity relationship cannot be
generalized for all commodities. Ahmed et al. (2009) reported that
hygroscopicity of spray-dried sweet potato was greatly affected by
carrier agents with no direct relationship to varying moisture
content. The present study was in agreement with his findings
wherein maltodextrin greatly influenced the hygroscopicity of
the spray-dried mango powder.
3.3. Glass transition temperature
The glass transition temperatures of RW-, freeze-, drum- and
spray-dried mango powders were determined in the water activity
range from 0.169 to 0.177 and water content below 0.05 kg water/
kg mango solids (Table 4
). The onset of T
g
(T
gi
) values of mango
powders were slightly lower than the room temperature (25 °C)
normally used for long-term storage of food powders. Adhikari
et al. (2009a) reported that water activity below 0.2 is the value
commonly applied for processing of spray-dried powders in a com-
mercial scale. The T
gi
values of mango powders ranged from
18.7 ± 0.2 to 26.1 ± 0.8 with RW-dried mango powder displaying
the lowest T
gi
and being significantly different from spray-dried
product having the highest T
gi
value (P < 0.05). The higher T
g
value
of spray-dried product may be attributed to the addition of higher
T
g
maltodextrin before spray drying. Maltodextrin has high molec-
ular weight and adding this amorphous material to low molecular
weight sugar-rich material such as mango will cause it to increase
the glass transition temperature of the product (Jaya and Das,
2004). The glass transition temperature of RW- freeze- and
drum-dried mango powder produced without the drying aids
showed no significant differences among each other (P < 0.05). It
can also be seen from the data that the glass transition
Table 3
Solubility and hygroscopicity of RW-, freeze-, drum-, and spray-dried mango powders with particle size 180–250
l
m.
Drying methods Particle size (
l
m) Moisture content (kg water/
kg mango solids)
Solubility
A
(%) Hygroscopicity
B
(%)
RW 180–250 0.017 ± 0.001 90.79 ± (0.394)
*,a
18.0 ± 0.36
a
FD 180–250 0.023 ± 0.002 89.70 ± 0.631
a
18.0 ± 0.19
a
DD 180–250 0.013 ± 0.001 94.38 ± 0.431
b
20.1 ± 0.88
b
SD 180–250 0.043 ± 0.003 95.31 ± 0.112
b
16.5 ± 0.06
c
*
Standard deviation from the average value.
a,b
Means with the same superscript letters within a column indicate no significant differences (P 6 0.05).
A
Measurement was done at 23 °C.
B
Samples were exposed to 75 ± 1% RH at 25 °C for 7 days.
Table 4
Glass transition temperatures and water activity of RW-, freeze-, drum-, and spray-
dried mango powders.
Drying methods Glass transition temperatures Water activity
T
gi
T
gm
T
gi
RW 18.7 ± 0.2
b
23.1 ± 0.7
a
24.6 ± 0.5
a
0.177 ± 0.001
a
FD 20.1 ± 0.8
ab
25.8 ± 2.7
a
27.6 ± 1.8
ab
0.174 ± 0.001
a
DD 22.4 ± 1.1
ab
27.6 ± 1.3
a
30.3 ± 1.1
ab
0.169 ± 0.002
a
SD 24.4 ± 0.8
a
28.8 ± 4.3
a
31.5 ± 0.7
a
0.173 ± 0.006
a
Means with the same superscript letters within a column indicate no significant
differences (P < 0.05). T
gi
, T
gm
and T
ge
represent the onset, mid- and end-point glass
transition temperatures of mango powders (180–250
l
m).
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
143
temperature of mango powder with water activity <0.2 was not af-
fected by the drying process and condition.
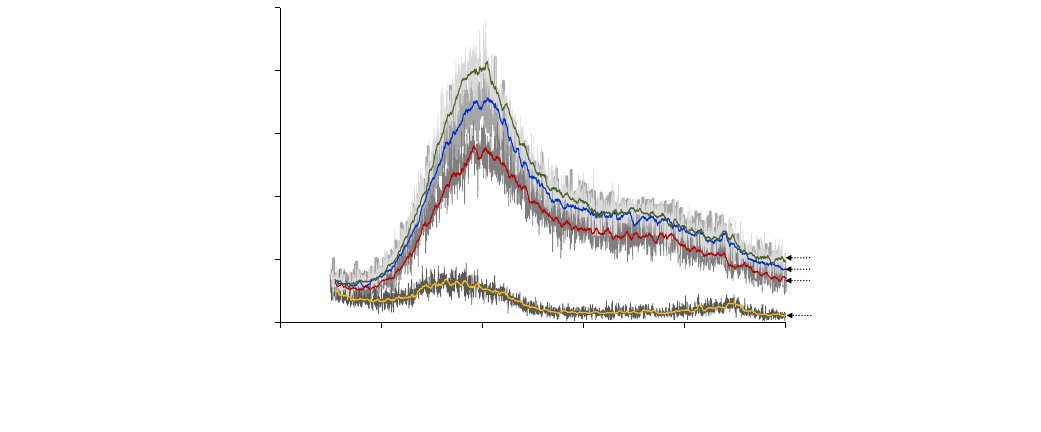
3.4. X-ray diffraction
X-ray diffraction is a common technique used to confirm the
crystalline–amorphous state of dried products in a powder form.
In general, crystalline material shows a series of sharp peaks, while
amorphous product produces a broad background pattern. The X-
ray diffraction patterns of RW-, freeze-, drum- and spray-dried
mango powders at a
w
< 0.2 clearly exhibited amorphous character-
istics and showed no crystalline peak formation (Fig. 7). The rapid
drying of low molecular weight sugars present in mango (sucrose,
fructose and glucose) and organic acids that happened under RW,
drum and spray drying processes tend to produce amorphous
metastable state dried products because of insufficient time to
crystallize (Jayasundera et al., 2011a,b). The diffractogram of
freeze-dried mango powder obtained in this study was similar to
the one reported by Harnkarnsujarit and Charoenrein (2011) and
Haque and Roos (2005).
The X-ray patterns and shapes for all the mango powders tested
were similar to spray-dried sucrose indicating the dominance of
sucrose sugars present in mango (Adhikari et al. (2009a). However,
it is interesting to note that the intensity count for drum-dried
mango powder as shown in the diffractograms was significantly
lower compared to the other three powder products. This could
be due to puree gelatinization that occurred before the actual drum
drying, resulting in the disorganization of intra- and intermolecu-
lar hydrogen bonding between water and starch molecules (Gavri-
elidou et al., 2002). Anastasiades et al. (2002) confirmed that
gelatinization process causes irreversible changes in the physical
structure of starch, which is present in mango resulting in degrada-
tion of molecular structure and loss of crystallinity. The absence of
crystalline peaks confirmed that no substantial changes occurred
on the hygroscopicity of RW-, freeze-, drum- and spray-dried man-
go powders as earlier discussed.
3.5. Microstructure
Scanning electron micrographic studies of mango powders
(180–250
l
m) obtained by different drying processes are shown
in Figs. 8–10. The microstructure of RW-dried mango powder
was smooth, and flaky with uniform thickness (Fig. 8a and b).
The uniformity of the flake thickness was the result of a controlled
feeding of mango puree using a spreader bar at the inlet section of
the RW dryer. During dying, the thinly spread mango puree on the
surface of the plastic film conveyor is undisturbed, except for re-
moval of moisture, as it moves toward the other end of the dryer,
hence producing a continuous sheet with thickness nearly equal.
Crushing the RW-dried mango flakes into powder form produced
irregularly shaped particles while maintaining its thickness. The
two sides of a single particle were smooth indicating more flow-
ability and less susceptibility to oxidation because of lesser surface
area. Freeze-dried mango powder (Fig. 8c and d), showed a skele-
tal-like structure and was more porous than the other mango pow-
ders. This result happens because the ice in the material during
freeze drying helps prevent shrinkage and collapse of the structure
and shape resulting in an insignificant change in volume (Ratti,
2001). The microstructure of drum-dried mango powder (Fig. 8e
and f) was compact and exhibited irregular particles with sharp
edges and considerable indentation as a result of crushing into
powder. Caric and Kalab (1987) reported similar structure for
drum-dried milk powder. They explained that the compactness
of drum-dried milk powder was due to deaeration of raw milk dur-
ing drum drying. It is also evident that the drum dried sheets are
smooth on one side that is in direct contact with the drum surface,
while visible corrugation and crinkle was observed on the other
side. These observations are in agreement with the microstructure
of drum-dried pre-gelatinized maize starches as described by
Anastasiades et al. (2002). Spray-dried mango powder (Fig. 8g
and h) has spherical or oval shape and smooth surface particles
due to effect of spray-drying condition, which was maintained at
inlet temperature of 190 ± 2 °C during drying. Nijdam and Langrish
(2005) demonstrated that milk powders spray-dried at inlet tem-
perature of 200 °C have spherical, smooth and larger particles,
while particles were smaller and shriveled when the inlet temper-
ature was reduced to 120 °C. The smooth spherical-shaped mango
powder contributed to its high porosity compared to the other
three drying methods.
Individual particles of mango powders obtained from different
drying processes were further examined (Fig. 9). The RW-dried
mango powder clearly showed a composite sheet with distinguish-
able internal pores within the particle indicating that some emp-
tied space during evaporation is not replaced as the mango puree
0
50
100
150
200
250
01020304050
Intensity (Counts)
2 Theta (Deg)
d
a
b
c
Fig. 7. X-ray diffraction patterns of Refractance Window
Ò
-dried (a), freeze-dried (b), drum-dried (c) and spray-dried (d) mango powders with particle size 180–250
l
m and
a
w
< 0.2.
144 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148

Fig. 8. Scanning electron micrographs (SEM) of mango powders (180–250
l
m) dried using Refractance Window
Ò
drying (a and b), freeze drying (c and d), drum drying (e and
f) and spray drying (g and h) (magnification of 100 (a, c, e and g) and 300 (b, d, f and h), 20 kV).
Fig. 9. Scanning electron micrographs (SEM) of individual mango powder particles (180–250
l
m) dried using Refractance Window
Ò
drying (a), freeze drying (b), drum drying
(c) and spray drying (d) (magnification of 1000, 20 kV).
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
145

is dried. These pores might have contributed to the higher porosity
of RW-dried compared to drum-dried mango powder. Drum-dried
mango powder developed a fine particle surface allowing it to be
more compact and rigid. Spray-dried mango powder particle
showed a very fine and smooth surface, but it may not be indica-
tive of being rigid and compact as it contains vacuoles forming a
hollow spherical shape (Cai and Corke, 2000). Apparently, external
pores were developed within the internal pores of a single particle
freeze-dried mango powder. This further explains why the porosity
of freeze-dried materials always is higher in comparison with other
drying methods.
The microstructures of mango powders (180–25
l
m) exposed
at 23 °C for 7 days at high relative humidity (75.5%) showed differ-
ent water adsorption behavior (Fig. 10). The particle surfaces and
edges of RW- and freeze-, and spray-dried mango powders were
still visible indicating that the materials adsorbed less when com-
pared to drum-dried mango powder wherein its particles were
nearly dissolved with water. This result confirmed the higher
hygroscopicity value obtained for drum-dried mango powder
compared to the other three powder products.
4. Conclusions
The physical properties and microstructures of mango powders
were significantly affected by drying methods applied. Drying of
mango puree to below 0.05 kg/kg dry mango solids was accom-
plished in 180 ± 0.15, 111,600 ± 5100 and 54 ± 0.2 s for RW, FD
and FD, respectively, and less than 3 s with SD. The color of
drum-dried mango powder was severely degraded because of high
processing temperature, while the spray-dried powder became
lighter due to the addition of maltodextrin. On the other hand,
the color of RW- and freeze-dried mango powder was comparable
at different particle sizes. The reconstituted RW-dried mango
puree showed a slight deviation in comparison with the original
puree and was very close to reconstituted freeze-dried mango
puree. Reconstituted drum- and spray-dried mango puree suffered
discoloration and were respectively darker and lighter than the ori-
ginal puree. Both the drum- and RW-dried mango powders were
significantly denser compared to freeze- and spray-dried. Regard-
less of the particle size and shape, freeze-dried mango powder
had the highest bulk porosity compared to the other three drying
methods. Drum-dried mango powder was the most hygroscopic
while spray-dried was the least hygroscopic. There was no signifi-
cant difference in hygroscopicity and solubility between RW and
freeze-dried material. The glass transition temperatures of RW-,
freeze-, drum- and spray-dried powders were not significantly dif-
ferent at water activity just below 0.2. The X-ray diffraction pat-
terns of RW-, freeze-, drum- and spray-dried mango powders
(a
w
< 0.2) clearly exhibited amorphous characteristics and showed
no crystalline peak formation. The microstructure analysis verified
the variations in bulk density, porosity, solubility and hygroscopic-
ity of mango powders. Also, the microstructures of individual par-
ticles played an important role in analyzing the physical properties
of mango powders. Overall, our study concludes that the RW dry-
ing method can produce superior quality mango powder compared
to drum and spray drying, while it is highly comparable to freeze
drying. The study provides an opportunity to the powder process-
ing industry in selecting a better drying technique that can be uti-
lized for the manufacture of high quality mango powder.
Acknowledgments
We thank the Ford Foundation International Fellowship Pro-
gram (IFP)/Institute of International Education (IIE)-New York
Fig. 10. Field emission scanning electron micrographs (FESEM) of Refractance Window
Ò
-dried (a), freeze-dried (b), drum-dried (c) and spray-dried (d) mango powders (180–
250
l
m) stored for 7 days at 25 °C with RH = 75.5% (magnification of 300, 30 kV).
146 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
through IFP-Philippine Social Science Council (IFP-PSCC) for pro-
viding the financial support, and the Philippine Center for Posthar-
vest Development and Mechanization (PhilMech) for granting
study leave to Ofero Caparino. Special thanks to Richard E. Magoon
and Karin M. Bolland of MCD Technologies, Inc (Tacoma, WA) for
allowing the use of their RW drying facilities, and for their assis-
tance in doing the experiments; Eng. Frank Younce and Ms. Galina
Mikhaylenko for assisting with the drying and physical analysis
experiments, respectively, Roopesh Syamaladevi for assisting in
X-ray diffraction, and Dr. Valerie Lynch-Holm for helping with
the SEM and FESEM imaging.
References
Abonyi, B.I., Feng, B.I., Edwards, C.G., Tang, J., 2002. Quality retention in strawberry
and carrot purees dried with Refractance Window system. Journal of Food
Science 67, 1051–1056.
Adhikari, B., Howes, T., Bhandari, B., Langrish, T.G., 2009a. Effect of addition of
proteins on the production of amorphous sucrose powder through spray drying.
Journal of Food Engineering 94 (2), 144–153.
Adhikari, B., Howes, T., Wood, B.J., Bhandari, B.R., 2009b. The effect of low molecular
weight surfactants and proteins on surface stickiness of sucrose during powder
formation through spray drying. Journal of Food Engineering 94, 135–143.
Ahmed, M., Akter, M.S., Eun, J.-B., 2010. Impact of alpha-amylase and maltodextrin
on physicochemical, functional and antioxidant capacity of spray-dried purple
sweet potato flour. Journal of Food Agriculture 90, 494–502.
Ameny, M.A., Wilson, P.W., 1997. Relationship between hunter color values and b-
carotene contents in white-fleshed African sweet potatoes (Ipomoea batatas
Lam). Journal of the Science of Food and Agriculture 73, 301–306.
Anastasiades, A., Thanou, S., Loulis, D., Stapatoris, A., Karapantsios, T.D., 2002.
Rheological and physical characterization of pregelatinized maize starches.
Journal of Food Engineering 52, 57–66.
AOAC, 1998. Official Methods of Analysis. Association of Official Analytical
Chemists, Washington, DC, USA.
Barbosa-Cánovas, G.V., 1996. Dehydration of Foods. Chapman and Hall, New York.
Barbosa-Canovas, G.V., Ortega-Rivas, E., Juliano, P., Yan, H., 2005. Food Powders:
Physical Properties, Processing, and Functionality. Kluwer Academic/Plenum
Publishers, New York.
BAS, 2009. Situation Report on Selected Fruit Crops. Retrieved March 1, 2011, from
<http://www.bas.gov.ph>.
Bhandari, B.R., Datta, N., Howes, T., 1997a. Problems associated with spray drying of
sugar-rich foods. Drying Technology 15 (2), 671–684.
Bhandari, B.R., Datta, N., Crooks, R., Howes, T., Rigby, S., 1997b. A semi-empirical
approach to optimize the quantity of drying aids required to spray dry sugar-
rich foods. Drying Technology 15 (10), 2509–2525.
Cai, Y.Z., Corke, H., 2000. Production and properties of spray-dried amaranthus
betacyanin pigments. Journal of Food Science 65 (6), 1248–1252.
Cano-Chauca, M., Stringheta, P.C., Ramos, A.M., Cal-Vidal, C., 2005. Effect of the
carriers on the microstructure of mango powder obtained by spray drying and
its functional characterization. Innovative Food Science and Emerging
Technologies 6, 420–428.
Caparino, O.A., 2000. Characteristics and quality of freeze-dried mango powder pre-
frozen at different temperatures. Philippine Agricultural Scientist Journal 83 (4),
338–343.
Caric, M., Kalab, M., 1987. Effects of drying techniques on milk powder quality and
microstructure: a review. Food Microstructure 6, 171–180.
Chen, X.D., Patel, K.C., 2008. Manufacturing better quality food powders from spray
drying and subsequent treatments. Drying Technology 26, 1313–1318.
Desai, K.G., Park, H.J., 2004. Solubility studies of valdecoxib in the presence of
carriers, co-solvent and surfactants. Drug Development Research 62, 41–48.
Desobry, S.A., Netto, F.M., Labuza, T.P., 1997. Comparison of spray-drying, drum-
drying and freeze-drying for b-carotene encapsulation and preservation. Journal
of Food Science 62 (6), 1158–1162.
Duffie, J., Marshall, W., 1953. Factors influencing the properties of spray-dried
materials. Chemical Engineering and Processing 49, 417–423.
Dziezak, 1988. Microencapsulation and encapsulated ingredients. Food Technology
42, 136–148.
Eastman, J.E., Moore, C.O., 1984. Cold Water Soluble Granular Starch for Gelled Food
Composition. U.S. Patent 4465702.
Evans, E.A., 2008. Recent Trends in World and US Mango Production Trade, and
Consumption. University of Florida. Food and Resource Economics Department,
Gainesville, Florida.
FAO, 2007. FAOSTAT Database Collections, Agricultural Data, Food and Agriculture
Organization of the United Nations. Retrieved 10.03.11, from <http://
www.faostat.org>.
Figiel, A., 2010. Drying kinetics and quality of beetroots dehydrated by combination
of convective and vacuum-microwave methods. Journal of Food Engineering 98,
461–470.
Filkova, I., Mujumdar, A.S., 1995. Industrial spray drying systems. In: Mujumdar,
A.S. (Ed.), Handbook of Industrial Drying. Marcel Dekker, New York, pp. 263–
307.
Gavrielidou, M.A., Vallous, N.A., Karapantsios, T.D., Raphaelides, S.N., 2002. Heat
transport to a starch slurry gelatinizing between the drums of a double drum
dryer. Journal of Food Engineering 54, 45–58.
Gonzalez-Aguilar, G.A., Zavaleta-Gatica, R., Tiznado-Hernandez, M., 2007.
Improving postharvest quality of mango ‘Haden’ by UV-C treatment.
Postharvest Biology and Technology 45, 108–116.
Goula, A.M., Adamopoulus, K.G., 2008. Effect of maltodextrin addition during spray
drying of tomato pulp in dehumidified air: II. Powder properties. Drying Journal
26, 726–737.
Haque, M., Roos, Y., 2005. Crystallization and X-ray diffraction of spray-dried and
freeze-dried amorphous lactose. Carbohydrate Research 340, 293–301.
Harnkarnsujarit, N., Charoenrein, S., 2011. Effect of water activity on sugar
crystallization and beta-carotene stability of freeze-dried mango powder.
Journal of Food Engineering 105, 592–598.
Hsu, C.L., Chen, W., Weng, Y.M., Tseng, C.Y., 2003. Chemical composition, physical
properties, and antioxidant activities of yam flours as affected by different
drying methods. Food Chemistry 83, 85–92.
Jakubczyk, E., Ostrowska-Ligeza, E., Gonde, E., 2010. Moisture sorption
characteristics and glass transition temperature apple puree powder.
International Journal of Food Science and Technology 45, 2515–2523.
Jaya, S., Das, H., 2004. Effect of maltodextrin, glycerol monostearate and tricalcium
phosphate on vacuum dried mango powder properties. Journal of Food
Engineering 63, 125–134.
Jaya, S., Das, H., Mani, S., 2006. Optimization of maltodextrin and tricalcium
phosphate for producing vacuum dried mango powder. International Journal of
Food Properties 9, 13–24.
Jayasundera, M., Adhikari, B., Adhikari, R., Aldred, P., 2011a. The effect of protein
types and low molecular weight surfactants on spray drying of sugar-rich foods.
Food Hydrocolloids 25 (3), 459–469.
Jayasundera, M., Adhikari, B., Adhikari, R., Aldred, P., 2011b. The effects of proteins
and low molecular weight surfactants on spray drying of model sugar-rich
foods: powder production and characterisation. Journal of Food Engineering
104 (2), 259–271.
Jayasundera, M., Adhikari, B., Howes, T., Aldred, P., 2011c. Surface protein coverage
and its implications on spray-drying of model sugar-rich foods: solubility,
powder production and characterisation. Food Chemistry 128 (4), 003–1016.
Kalogiannia, E.P., Xynogalos, V.A., Karapantsios, T.D., Kostloglou, M., 2002. Effect of
Feed Concentration on the Production of Pregelatinized Starch in a Double
Drum Dryer. Elsevier Science Ltd.
Karel, M., 1975. Freeze dehydration of foods. In: Karel, M., Fennema, O.R., Lund,
D.B. (Eds.), Principles of Food Science. Marcel Dekker, Inc., New York,
pp. 359–395.
Kha, T.C., Nguyen, M.H., Roach, P.D., 2010. Effects of spray drying conditions on the
physicochemical and antioxidant properties of the Gac (Momordica
cochinchinensis) fruit aril powder. Journal of Food Engineering 98, 385–392.
Kim, E.H.-J., Chen, X.D., Pearce, D., 2009. Surface composition of industrial spray-
dried milk powders. 2. Effects of spray drying conditions on the surface
composition. Journal of Food Engineering 94, 169–181.
Krokida, M.K., Maroulis, Z.B., 1997. Effect of drying method on shrinkage and
porosity. Drying Technology 15 (10), 2441–2458.
Krokida, M.K., Maroulis, Z.B., Saravakos, G.D., 2001. The effect of the method of
drying on the color of dehydrated products. International Journal of Food
Science and Technology 36, 53–59.
Masters, K., 1985. Spray Drying Handbook, fourth ed. Godwin, London.
Moore, J.G., 2005. Drum dryers. In: Mujumdar, A.S. (Ed.), Handbook of Industrial
Drying, vol. 1, second ed., Marcel Dekker, New York, pp. 249–262.
Mujumdar, A.S., 2007. Handbook of Industrial Drying, third ed. Taylor and Francis
Group, CRC Press, Florida.
Niamnuy, C., Nachaisin, M., Laohavanich, J., Devahastin, S., 2011. Evaluation of
bioactive compounds and bioactivities of soybean dried by different methods
and conditions. Food Chemistry, 899–906.
Nijdam, J.J., Langrish, T.A., 2005. An investigation of milk powders produced by a
laboratory-scale spray dryer. Drying Technology 23 (5), 1049–1056.
Nindo, C.I., Tang, J., 2007. Refractance Window dehydration technology: a novel
contact drying method. Drying Technology 25, 37–48.
Nindo, C.I., Sun, T., Wang, S.W., Tang, J., Powers, J.R., 2003. Evaluation of drying
technologies for retention of physical and chemical quality of green asparagus
(Asparagus officinalis, L.). Food Sci. Technol. (LWT) 36 (5), 507–516.
Oetjen, G.W., Haseley, P., 2004. Freeze-drying, second ed. Wiley-VCH GmbH & Coo.
KGaA, Germany.
Potter, N.N., Hotchkiss, J.H., 1995. Food Science, fifth ed. Springer, New York.
Rajkumar, P., Kailappan, R., Viswanathan, R., Raghavan, G.S., Ratti, C., 2007. Foam
mat drying of Alphonso mango pulp. Drying Technology 25, 357–365.
Ratti, C., 2001. Hot air and freeze-drying of high-value foods – a review. Journal of
Food Engineering 49, 311–319.
Rodríguez-Hernández, G.R., Gonzalez-Garcia, R., Grajales-Lagunes, A., Ruiz-Cabrera,
M.A., 2005. Spray-drying of cactus pear juice (Opuntia streptacantha): effect on
the physicochemical properties of powder and reconstituted product. Drying
Technology 4, 955–973.
Sablani, S.S., Shrestha, A.K., Bhandari, B.R., 2008. A new method of producing date
powder granules: physicochemical characteristics of powder. Journal of Food
Engineering 87 (3), 416–421.
Saravacos, G.D., Kostaropoulos, A.E., 2002. Handbook of Food Processing Equipment.
Kluwer Academic/Plenum Publishers, New York.
Serrano, E.P., 2005. Postharvest management of fruit and vegetables in the Asia-
Pacific region: Philippines country report. Asian Productivity Organization
O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
147
(APO); Food and Agriculture Organization of the United Nations (FAO). APO and
FAO, Japan, Rome, pp. 216–226.
Shrestha, A.K., Ua-arak, T., Adhikari, B.R., Howes, T., Bhandari, B.R., 2007. Glass
transition behavior of spray-dried orange juice powder measures by differential
scanning calorimetry (DSC) and thermal mechanical compression test (TMCT).
International Journal of Food Properties 10, 661–673.
Syamaladevi, R.M., Sablani, S.S., Tang, J., Powers, J., Swanson, B.G., 2009. State
diagram and water adsorption isotherm of raspberry (Rubus idaeus). Journal of
Food Engineering, 460–467.
Toledo, R., 2007. Fundamentals of Food Process Engineering, 3rd ed. Aspen
Publishers, Inc., Athens, GA, USA.
Tonon, V.R., Brabet, C., Hubinger, M.D., 2008. Influence of process conditions on the
physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced
by spray drying. Journal of Food Engineering 88, 411–418.
Wagner, L.A., Warthesen, J.J., 1995. Stability of spray-dried encapsulated carrot
carotenes. Journal of Food Science 60, 1048–1053.
Wang, S., Konkol, E., Langrish, T.G., 2011. Spray drying of fruit juice using proteins as
additives. Drying Technology 29 (16), 1868–1875.
Xiaoyan, Z.Y., 2008. Effect of superfine grinding on properties of ginger powder.
Journal of Food Engineering 91 (2), 217–222.
148 O.A. Caparino et al. / Journal of Food Engineering 111 (2012) 135–148
