
Permit Guidance 5 Biomonitoring Test Guidance July 98 Page 1
Permit
Guidance
5
Final
Reporting and Testing Guidance for Biomonitoring
Required by the Ohio Environmental Protection Agency
Rule reference: 40CFR Part 136.3;
OAC 3745-2-09; OAC 3745-33-07
Ohio EPA, Division of Surface Water
Revision 0, October 1991
Revision 1, July 1998
This internal guidance does not affect the requirements found in the referenced rule or statute.
Purpose
This document provides detailed instructions to Ohio EPA staff and other professionals who
collect and analyze water samples for biomonitoring (bioassays) required by NPDES permits in
Ohio.
Background
The manual is intended to provide detailed information on the most frequently used procedures,
methods, quality assurance practices and data reporting techniques for bioassay tests.
Procedure
The report is attached. Contact the office listed below for more information.
Related Policy or guidance
Ohio EPA. 1995. Manual of Ohio EPA Laboratory Standard Operating Procedures.
Volumes I, II, III and IV.
For more information contact:
Ohio EPA, Division of Surface Water
Industrial Permit Group Leader
(614) 644-2001
C:\Documents and Settings\rheitzma\My Documents\permit5.wpd


TABLE OF CONTENTS
Page No.
Introduction ..................................................................................................... 1
Section 1: Mandatory Requirements for NPDES Biomonitoring
A. Use of Approved Methods ........................................................................ 2
B. Standard Operating Procedures ................................................................. 3
Section 2: Requirements for Acute Toxicity Testing
A. Acute Toxicity as an Effluent Characteristic ............................................ 4
B. Test Organisms .......................................................................................... 4
C. Length of Acute Toxicity Tests ................................................................. 4
D. Type of Test .............................................................................................. 5
E. Sample Collection ...................................................................................... 6
F. Quality Assurance ...................................................................................... 7
G. Chemical Analysis ..................................................................................... 8
H. Reporting Requirements ............................................................................ 9
Section 3: Requirements for Chronic Toxicity Testing
A. Chronic Toxicity as an Effluent Characteristic ......................................... 14
B. Test Organisms .......................................................................................... 15
C. Length of Chronic Toxicity Tests .............................................................. 15
D. Type of Test ............................................................................................... 15
E. Sample Collection ...................................................................................... 17
F. Quality Assurance ...................................................................................... 18
G. Chemical Analysis ..................................................................................... 19
H. Reporting Requirements ............................................................................ 20
Section 4: Requirements for Instream Biosurveys
A. Function of Instream Biological Surveys .................................................. 26
B. Factors Examined in Conducting a Biosurvey .......................................... 26
C. Time Period for Performing Survey Work ................................................ 27
D. Type of Survey .......................................................................................... 27
E. Sample Collection ...................................................................................... 27
F. Study Plan Submission .............................................................................. 27
G. Sampling of Ambient Waters for Instream Biosurveys ............................ 28
H. Reporting Requirements ............................................................................ 28
I. Collection Permit ........................................................................................ 30
Section 5: NPDES Permit Application Requirements
A. Guidelines for Submittal of This Data ...................................................... 30
Attachment 1: Form 4500
Attachment 2: Example Form 4500 Showing Acute Toxicity Test Results
Attachment 3: Ohio EPA NPDES Biomonitoring Report Form, Acute Toxicity Test
Attachment 4: Example Form 4500 Showing Chronic Toxicity Test Results
Attachment 5: Ohio EPA NPDES Biomonitoring Report Form, Chronic Toxicity Test
Attachment 6: Biosurvey Sampling Information

1
REPORTING AND TESTING GUIDANCE FOR BIOMONITORING
REQUIRED BY OHIO ENVIRONMENTAL PROTECTION AGENCY
Introduction
This document has been prepared to provide guidance on the reporting and testing requirements
for biomonitoring conditions in a National Pollutant Discharge Elimination System (NPDES)
permit. It includes important changes to reporting procedures for biomonitoring, as well as
clarification of test procedures.
Biomonitoring is designed to evaluate the impact or potential impact of a wastewater discharge
on aquatic life using biological methods. Biomonitoring requirements may come in two basic
forms: 1) evaluating effluent toxicity through toxicity tests; and 2) evaluating the impact of an
effluent through assessment of the instream community.
Toxicity testing uses aquatic organisms to directly measure effluent toxicity and is the most
common form of biomonitoring included in NPDES permits. Typically, NPDES permits in Ohio
will require toxicity testing using fathead minnows (Pimephales promelas) and Ceriodaphnia
dubia, although Ohio EPA will consider the use of alternative test species. Prior approval by
Ohio EPA is required for use of alternative test species.
There are three methods of effluent toxicity testing, flow-through, static, and static renewal. A
flow-through toxicity test requires that the tested effluent be constantly pumped through the test
chamber. A static test requires that the sample used to initiate the test is the only one used for
the duration of the test. A static renewal test requires that the test solutions be renewed daily
using the original sample or additional samples collected during the testing period.
Toxicity tests may also measure different types of effects depending upon the duration and intent
of the test. Acute toxicity tests measure short-term, obvious effects. Chronic toxicity tests
measure longer-term, but potentially more subtle effects. The difference between these two
types of tests involve the duration of the test and the toxicity end points measured in the tests.
An acute toxicity test usually has a duration of 2 to 4 days, depending upon the test species. The
end points measured are death or atypical behavior or appearance. A chronic toxicity test may
last as long as 1 year or more. Early life stage chronic tests may last 28 to 30 days. Typically, a
chronic or sub-chronic toxicity test required by an NPDES permit is for the shorter term of 7
days. The end points measured are growth or reproductive effects, as well as death and/or
atypical behavior or appearance.
The second form of biomonitoring is the instream community assessment. It is a direct measure
of the structure and function of the aquatic community living in the receiving water. The
assessment is made by sampling the resident populations and comparing existing aquatic

2
populations to the criteria for minimally-impacted aquatic communities established in the Ohio
Water Quality Standards (WQS).
Ohio EPA may require some or all of the above testing requirements based upon factors that
apply to a particular facility. This document identifies the specific methods to be used in
conducting biomonitoring programs and provides guidance on how to fulfill reporting
requirements of the NPDES permit.
Section 1: Mandatory Requirements for NPDES Biomonitoring
A. Use of Approved Methods.
The use of Ohio EPA-approved methods is required for biomonitoring conducted pursuant
to the terms of an NPDES permit. Alternative methods may be used only after obtaining
prior approval from Ohio EPA and USEPA. In order to solicit approval for an alternative
method, the entity should submit a written request to:
Ohio EPA - Division of Surface Water
P.O. Box 1049
Columbus, Ohio 43216-1049
The following are approved test methodologies for biomonitoring being conducted as a
required of an NPDES permit
1. Approved Methods for Toxicity Testing:
The following documents contain methods that are approved for use in conducting
effluent or other toxicity testing:
a. 40 CFR 136.3 (Tables 1A and II).
Methods to Measure the Acute Toxicity of Effluent and Receiving Waters To
Freshwater, Estuarine and Marine Organisms
Short-Term Methods to Estimate the Chronic Toxicity of Effluents and
Receiving Waters to Freshwater Estuarine and Marine Organisms
b. Ohio EPA Quality Assurance Manual (current edition).
These documents can be obtained by contacting Ohio EPA-Division of
Environmental Services, Bioassay Section, P.O. Box 1049, Columbus, Ohio 43216-
1049.

3
2. Approved Methods for Instream Biological Assessment:
The following documents contain methods that are approved for use in conducting stream
surveys to demonstrate attainment of biological criteria established in the Ohio WQS (Ohio
Administrative Code (OAC) 3745-1-07. All of the following documents are required to be
utilized in the design, execution and reporting of biomonitoring conducted for comparison
with the biocriteria contained in OAC 3745-1-07:
a. Ohio EPA Surveillance Methods and Quality Assurance Manual (current edition).
b. Biological Criteria for the Protection of Aquatic Life: Volume I. The Role of
Biological Data in Water Quality Assessment, Ohio EPA, 1987.
c. Biological Criteria for the Protection of Aquatic Life: Volume II. Users Manual for
Biological Field Assessment of Ohio Surface Waters, Ohio EPA, 1987.
d. Addendum to Biological Criteria for the Protection of Aquatic Life: Volume II.
Users Manual for Biological Field Assessment of Ohio Surface Waters, Ohio EPA,
1989.
e. Biological Criteria for the Protection of Aquatic Life: Volume III. Standardized
Biological Field Sampling and Laboratory Methods for Assessing Fish and
Macroinvertebrate Communities, Ohio EPA, 1989.
f. The Qualitative Habitat Evaluation Index (QHEI): Rationale, Methods and
Application, Ohio EPA, 1989.
These documents can be obtained by contacting Ohio EPA-Division of Surface Water, P.O.
Box 1049, Columbus, Ohio 43216-1049.
B. Standard Operating Procedures.
Whether the permittee performs the biomonitoring work or retains a contract laboratory or
consulting firm to perform the necessary testing, it is necessary to submit an official
Standard Operating Procedure (SOP) to Ohio EPA. The SOP is a detailed explanation of
the actual techniques used to conduct tests required by NPDES permits. The submission of
an SOP will enable Ohio EPA to minimize the reporting requirements necessary for each
test result, as well as verify that approved test methodologies are being utilized.
If the permittee chooses to retain a contract laboratory, a previous SOP submittal will be
sufficient to fulfill this condition, so long as the SOP accurately reflects the laboratory’s
current operations. If an SOP has not been submitted, the permittee is responsible for
assuring the timely submittal of an SOP by the laboratory performing the testing. If an

4
SOP has previously been submitted, the permittee should notify Ohio EPA at the address
below, indicating the date and title of the submittal. Any necessity to deviate from the SOP
due to special case considerations should be noted as such on the test report. Ohio EPA
should be consulted prior to conducting the test if a reason for deviating from the SOP is
known to exist at that time. Ohio EPA reserves the right to comment on an SOP
concerning issues perceived to be in conflict with approved methods.
SOP’s should be submitted, in duplicate, to:
Permits Section
Ohio EPA-Division of Surface Water
P.O. Box 1049
Columbus, Ohio 43216-1049
Section 2: Requirements for Acute Toxicity Testing
This Section contains NPDES permit requirements for effluent toxicity testing designed to
measure acute toxicity. These requirements should be followed unless specifically modified by
the NPDES permit.
A. Acute Toxicity as an Effluent Characteristic.
Acute toxicity in an effluent toxicity test is measured as a short-term effect induced by
exposure to an effluent. The end points for an acute toxicity test are generally death of the
organism and/or atypical behavior or appearance (which is termed an effect). Summary
statistics, such as median lethal concentration (LC
50
) or percent morality, are determined
following completion of the test.
B. Test Organisms.
Test organisms used for toxicity testing required by NPDES permits will be Pimephales
promelas (fathead minnow) and Ceriodaphnia dubia. Additional test species may be
required based upon site-specific factors.
Alternative test species may be used for toxicity testing only with the prior approval of
Ohio EPA.
C. Length of Acute Toxicity Tests.
Acute toxicity tests will be for the durations specific for the following organisms:

5
1. Ceriodaphnia dubia: Acute toxicity tests using Ceriodaphnia dubia will be 48 hours in
duration. Test organism survival and observations of behavior and external
appearance shall be recorded every 24 hours at a minimum.
2. Pimephales promelas: Acute toxicity tests using fathead minnows will be 96 hours in
duration except as indicated in Section 2.D.1.a. Test organism survival and
observations of behavior and external appearance shall be recorded every 24 hours at
a minimum.
D. Type of Test.
Acute toxicity tests required by NPDES permits or Director’s Final Findings & Orders
(DFFO’s) will be static tests. Static renewal or flow-through tests may be performed only
as approved or required by Ohio EPA.
Acute toxicity tests may be categorized as either screening tests or definitive tests. A
screening test is a test in which the effluent and control solutions are evaluated at full
strength. This is an inexpensive test designed to indicate presence or absence of toxicity in
the effluent. A definitive test uses dilutions of the effluent to quantify toxicity. These two
types of tests are described further below:
1. Acute Screening Tests: Ohio EPA uses screening toxicity tests of different durations
depending upon the intended use of the toxicity data. These are described below:
a. General Screening Tests - These tests are performed by Ohio EPA or the
permittee to screen for toxicity in the effluent. The effluent and control
solutions are tested full strength, but the test durations are 48 hours for both
Pimephales promelas and Ceriodaphnia dubia.
b. Additive Screening Tests - These tests may be required to be conducted as a
condition of an Ohio EPA Administrative Order (DFFO’s) approving the use of
a cooling water additive. The effluent and control solutions are tested full
strength and test durations are 96 hours for Pimephales promelas and 48 hours
for Ceriodaphnia
dubia.
2. Acute Definitive Tests: A definitive test is designed to quantify the amount of
toxicity in an effluent. In order to accomplish this, the effluent is diluted to various
concentrations with one of the control waters. A minimum of 5 effluent
concentrations shall be used in a definite test. The typical dilutions used are 100, 50,
25, 12.5 and 6.25 percent by volume effluent.
There may be instances when it will be advantageous to use a different dilution
series. Selection of the appropriate dilution series is the responsibility of the
6
permittee. Ohio EPA staff will be available for consultation on the issue of
appropriate dilution series. However, the permittee, in conjunction with the testing
laboratory, may exercise professional judgement on the selection of appropriate
dilution series. When selecting a dilution series, the following should be considered:
a. Allowable Effluent Toxicity (AET) - AET is the permissible amount of toxicity
for a particular discharge. This value is determined by factoring the available
dilution in the receiving stream with the water quality criteria for toxicity as
well as the effects of any interactive discharges. Should permit limitations for
toxicity be established for a permittee, the AET is the amount of toxicity that
would be allowed. The method for determining the AET is established in OAC
3745-2-09. AET values are also given in NPDES fact sheets and wasteload
allocations. When selecting a dilution series, the relationship of the data
obtained through use of that series to the AET should be considered. The series
selected should be one which will yield data to determine if the AET has/has
not been exceeded, yet still identifies the LC
50
.
b. Toxicity in the Effluent - There may be instances where it is necessary to alter
the dilution series in order to better determine an LC
50
. This would be the case
in an effluent that is moderately acutely toxic. In order to give a better estimate
of the LC
50
, the dilution series has to be shifted to a higher set of dilutions.
Also, if effluent toxicity is consistently exhibited within a certain range, it may
be advisable to adjust the dilution series to focus in that area to better define the
LC
50
value.
For example, an effluent has an LC
50
of roughly 78 percent effluent and the
dilution series used in testing is 100, 50, 25, 12.5 and 6.25 percent volume by
effluent. Test results would often turn up as 100 percent morality in 100
percent effluent, and no effect in 50 percent effluent. If the effluent is not too
variable, the dilution series should be shifted upward to better characterize the
apparent LC
50
of 78 percent effluent. A dilution series of 100, 75, 50, 25 and
12.5 percent effluent by volume might be used in this instance, to better define
the LC
50
.
E. Sample Collection.
Effluent samples used to conduct the acute toxicity tests shall be collected as 24-hour
composite samples. If the effluent is chlorinated for disinfection purposes, the effluent
sample should be collected at a point prior to chlorination. The protocols in Section 1.A.1
should be consulted for the handling of a chlorinated sample if it is impossible to obtain a
sample prior to disinfection. However, if dechlorination is an integral part of the
disinfection system at the facility, the sample should be collected at the final outfall.

7
Unless specifically modified by the NPDES permit, an acute toxicity test of an effluent
requires that an upstream control and a near-field downstream sample be collected. The
upstream control sample is to be collected as a grab sample upstream from the zone of
effluent and receiving water interaction. Care should be taken to assure than any upstream
backflow of the effluent is taken into account when selecting the upstream sampling
location.
The near-field downstream sample is to be collected as a grab sample from within the
effluent plume in the immediate vicinity of the outfall. The near-field sample should be
collected in the middle of the effluent plume at a distance of 5 times the water depth at the
point of discharge, down current from the outfall. When water depth at the point of
discharge exceeds 4 feet, the near-field sample should be collected 20 feet (6 meters) down
current from the outfall.
The location of the near-field sample with respect to the effluent plume must be determined
and documented at the time of sampling using temperature measurements, conductivity
measurements, visual observation, a dye study, or other detailed techniques for delineating
the effluent plume.
Samples taken for toxicity testing purposes must be used within 36 hours after completion
of sample collection.
F. Quality Assurance.
1. Requirements for the Repeating of a Test:
There may be instances when poor survival or other adverse effects exhibited by
control organisms preclude the use of data from an effluent toxicity test and the test
must be repeated. The following conditions outline when a repeat of an acute toxicity
test is mandatory. Failure to repeat a toxicity test when necessary may result in
monitoring frequency violations of NPDES permit conditions.
A repeat of an acute toxicity test is mandatory when a combination of mortality and
adverse effects in both
receiving water and laboratory controls exceeds 10 percent of
a particular species. A repeat test is not necessary if there is 10 percent or less
affected in one of the two control waters. A repeat test is necessary only for the
species exhibiting unacceptable effects in the controls.
Failure to follow approved procedures may result in a requirement to repeat a toxicity
test. Any deviations from approved procedures should be explicitly described in the
report of the tests results.
2. Dilution Water Substitution:

8
If a combination of mortality and adverse effects in the upstream control sample
exceeds 10 percent for a particular test organism in a sample, an alternative dilution
and control water should be identified and used for subsequent tests. Acceptable
alternative dilution waters may be similar natural waters, rearing unit water,
reconstituted water, or dilute mineral water. An upstream receiving water sample
must still be collected and tested in subsequent tests, but shall not be used as diluent.
Failure to change the dilution water in subsequent tests, following a test with
excessive mortality in the receiving water control, may result in invalidation of
testing results and a requirement to repeat the test.
3. Number of Organisms:
At least 20 organisms of a particular species shall be used for each solution tested in
an acute toxicity test.
4. Test Temperature:
The approved methods for acute toxicity listed in Section 1.A.1 contain different test
temperatures for the species depending upon the purpose and type of tests to be
conducted. For data comparability and uniformity between acute and chronic tests, a
test temperature of 25
o
+ 1
o
C should be used in acute tests. The test temperature that
will be used should be listed in the SOP.
5. Feeding During Testing:
The U.S. EPA guidance manual for acute toxicity testing recommends feeding C.
dubia during an acute toxicity test if the solutions are renewed at 48-hours of
exposure. However, due to the fact that Ohio EPA is specifying static acute tests, we
recommend that the organisms should not be fed during the test. The pool of
organisms that are to be used for the tests should be fed while in holding immediately
prior to test initiation. A minimum amount of this water should be transferred to the
test solutions.
G. Chemical Analysis:
A sufficient volume of effluent shall be collected to allow aliquots for use in acute toxicity
tests and chemical analysis. Bioassay effluent sampling may be coordinated with other
permit sampling requires as appropriate to avoid duplication. The analyses detailed in the
currently effective Part I, Effluent Limitations and Monitoring Requirements tables in the
NPDES permit must be conducted for the effluent sample. In addition, alkalinity and
hardness (as CaCO
3
) should be measured. Chemical analysis must comply with Ohio EPA
accepted procedures.

9
H. Reporting Requirements:
1. Reporting Toxicity Testing Results on Monthly Operating Reports - NPDES permits
require that results of testing be reported on a form acceptable to Ohio EPA. The
results of toxicity tests required under Part I Permit Requirements shall be reported
on EPA Form 4500. A copy of Form 4500 is included as Attachment 1.
Results for final effluent toxicity shall be expressed as toxic units on Form 4500. An
acute toxic unit (TU
a
) is defined as:
TU
a
= 100
LC
50
Form 4500 should be received by Ohio EPA Central Office by the 15th day of the
month following the month in which the toxicity test samples were collected. It may
be necessary to obtain or provide the toxic unit results prior to receipt of the full
laboratory report in order to fulfill this requirement. The toxicity test result should be
recorded on the first day of sampling for the toxicity test.
For purposes of reporting on Form 4500 only, the following conventions should be
used to report effluent toxicity tests where an LC
50
cannot be identified:
Percent Mortality or Other Adverse
TU
a
Effect in Whole (100%) Effluent
0.9 45
0.8 40
0.7 35
0.6 30
0.5 25
0.4 20
0.3 15
0.2 10
AA (below detectable) <10
The above conventions should be used for reporting valid toxicity tests (i.e., tests that
satisfy the requirements of Section 2.F). If a test is not valid due to control mortality
or other problems, the code “AE” (analytical data not valid) should be used to report
the test results on Form 4500. The test should then be repeated as appropriate.
Reporting for instream stations shall be in percent of organisms affected. This
percentage should reflect all mortality and atypical behavior or appearance by the test

10
organisms. Results from upstream stations (those NPDES monitoring stations
numbered as 801, 802, etc.) should be reported as the percent affected from that
sample. In the event that an alternative control/dilution water is used, data from the
upstream ambient station (if required) should still be reported and the fact that an
alternative dilution water was used should be recorded in the comments section on
the form. Downstream stations (those NPDES monitoring stations numbered as 901,
902, etc.) should reflect the results of the near-field sample. If no mortality or effects
are observed in these solutions, record “AA” (below detectable) on the 4500 form.
See Attachment 2 for an example of a completed Form 4500 showing the results of an
acute toxicity test.
Timely submittal of these data allows for input of toxicity testing results into Ohio ‘s
mainframe computer systems.
2. Information Reported for Acute Toxicity Tests:
Ohio EPA has established two basic reporting formats for toxicity testing results,
depending upon whether the permittee has effective toxicity unit (TU
a
) limitations in
its NPDES permit.
a. Reporting for Permittees with Detailed Reporting Requirements - If the
permittee does not have effective toxic unit limitations in its permit, additional
information shall be submitted, as well as the necessary reporting on Form
4500. Reports containing the additional information for acute toxicity
monitoring requirements shall be submitted to the following address within 60
days of the initiation of the test:
Permits Section
Ohio EPA-Division of Surface Water
P.O. Box 1049
Columbus, Ohio 43216-1049
Reporting of acute toxicity test results shall be submitted on the “Ohio EPA
NPDES Biomonitoring Report Form” (see Attachment 3). The biomonitoring
report consists of the following five parts: 1) General Information; 2) Acute
Toxicity Test Sampling Data; 3) Toxicity Test Conditions; 4) Acute Toxicity
Test Results and 5) Additional Toxicity Test Information and
Conclusions/Comments. The following is guidance on completion of the five
portions of the biomonitoring report:
1. General Information - Much of the information in this part is self-
explanatory. However, additional details follow for some items:
11
o
Reporting Date is the date the report is submitted
o
SOP’s are the standard operating procedures required by the NPDES
permit and described in Section 1.B. An SOP need only be submitted
once for any particular testing laboratory; the SOP on file with Ohio
EPA must be accurate and current. If the testing laboratory has
previously submitted an SOP, mark “yes” and indicate the date the SOP
was submitted.
o
The Certification Statement and Signature form must be signed by a
responsible person in charge of the facility as defined by 40 CFR
122.22.
2. Acute Toxicity Test Sampling Data - Describe conditions relating to the
collection of samples to be tested. This part should be filled out as
necessary for each outfall that is tested. The information entered in this
part of the form is self-explanatory.
3. Toxicity Test Conditions - Describe the conditions during the toxicity test.
This part should be filled out for each species tested. Additional details
describing some of the information requested are listed below:
o
For Test Type and Duration, the test type would typically be static;
durations should be listed in hours.
o
The Light Quality states the type of lighting system and/or light
intensity, if available.
o
For Dilution and Primary Control Water, list the source of the dilution
and primary control water (e.g., receiving water, rearing unit water,
dilute mineral water, etc.).
o
The Secondary Control Water lists the source of the secondary control
water (e.g., hard reconstituted water, receiving water, dilute mineral
water, etc.).
o
For aeration, indicate whether or not the sample required aeration and if
aeration was required before or during the test.
4. Acute Toxicity Test Results - Describe the results of the acute toxicity
test. This part should be filled out for each species tested and for each
outfall tested. Much of the information that is requested is self-
explanatory. Additional details describing some of the information
requested are listed below:
12
o
Cumulative Percent Mortality is the cumulative total of dead organisms,
expressed as a percentage of the total test organisms for that solution
recorded every 24 hours. The result should be recorded on the line
corresponding to the solution that was tested.
o
Cumulative Percent Affected is the cumulative total of organisms
showing some adverse effect (e.g., death, disorientation, atypical
appearance or behavior), expressed as a percentage of the total test
organisms for that solution recorded every 24 hours. The result should
be recorded in the parentheses below the line showing percent mortality
at the time for that solution.
o
For Method(s) Used to Determine ..., list the graphical or statistical
methods used to derive the LC
50
, and their 95 percent confidence limits.
5. Additional Toxicity Test Information - This portion is used to present
information about the effluent plume during sampling, as well as any
conclusions that may be drawn from the data, and should be completed for
each species and outfall. Additional details on the information requested
are listed below:
o
For Method(s) Used to Verify Near-Field and/or Far-Field Sampling
Locations ..., complete this portion indicating the method used to verify
the location of the effluent plume during the sampling (e.g.,
conductivity, temperature, visual, etc.).
o
Conclusions/Comments should be completed for each species and
outfall tested as the permittee or testing laboratory as deemed
appropriate. The information listed under Additional Toxicity Test
information must be attached to the report.
b. Reporting for Permittees with Toxic Unit Limits - If the permittee has effective
toxic unit permit limitations, the only reporting required is on Form 4500,
provided that the SOP requirements (see Section 1.B) has been fulfilled. All
other data elements required by this Section (see items 1 through 21, below)
shall be retained by the permittee in accordance with Part III.7, Records
Retention, of the NPDES permit. Although records retention requirements
specify that records be maintained for 3 years, Ohio EPA recommends that
toxicity test information be retained for 5 years (or through the subsequent
permit renewal).
13
The following information is required to be retained as supporting information
for each test. This data will be used, if necessary, to assure the validity of data
submitted on Form 4500.
1. Name and address of the testing laboratory.
2. Name and address of the facility which is the source of the effluent tested.
3. Ohio EPA and NPDES permit numbers for the facility.
4. Receiving water tested and/or used.
5. Source of dilution water.
6. Date and time of sample collection.
7. Collector(s) name(s).
8. Type of toxicity test.
9. Test organisms used.
10. Test organism origin and acclimation process.
11. Number of organisms per container and per concentration.
12. Test container size, number per concentration, and depth of test solution.
13. Concentrations tested and volume.
14. Test temperature.
15. Results of chemical analyses.
16. Results of physicochemical measurements taken during the test.
17. Definition of adverse effects measured in the test (end points).
18. Number of organisms in each concentration showing the adverse effects at
specified times.
14
19. Median lethal concentrations (LC
50
) for each 24-hour interval during the
test, confidence limits for those values, and the methods used for the
calculations.
20. Exact details for the near-field sample location in relation to the outfall
and plume location along with the results from temperature, conductivity
or dye study used to confirm the effluent plume. If conducted, results of
the detailed mixing zone study shall be provided.
21. Any other relevant information.
Section 3: Requirements for Chronic Toxicity Testing
This Section discusses the details of NPDES permit requirements for effluent toxicity testing
designed to measure chronic toxicity. These requirements should be followed unless specifically
modified by the NPDES permit.
A. Chronic Toxicity as an Effluent Characteristic.
Chronic toxicity in an effluent toxicity test is measured as an effect or effects induced by a
relatively long-term exposure to an effluent. The end points for a chronic toxicity test are
growth or reproductive effects, as well as death of an organism and atypical appearance or
behavior. End results of chronic toxicity tests are defined by the Lowest Observed Effect
Concentration (LOEC), the No Observed Effect Concentration (NOEC), and the Inhibition
Concentration (IC). The IC is the concentration that would cause a given percent reduction
in a non-quintal biological measurement (e.g., the IC25 would cause a 25 percent reduction
in mean young Ceriodaphnia dubia reproduction or in Pimephales promelas growth, and
the IC50 would cause a 50 percent reduction in reproduction or growth). A non-quintal
effect is an all or nothing response (e.g., life vs. death, or motile vs. immotile). The LOEC
is the lowest concentration in which a particular effect (e.g., reduced growth, reproduction
or survival) is exhibited at a statistically significant level. The NOEC is the highest
concentration in which no statistically significant effects are observed.
The IC25 is determined by statistical point estimation techniques and may be accompanied
by confidence limits. The NOEC and LOEC are determined by statistical hypothesis testing
techniques and are dependent upon the dilution factor used in a toxicity test. Statistical
confidence limits may not be placed about the NOEC or LOEC.
B. Test Organisms.
The test organisms used for chronic toxicity testing will be Ceriodaphnia
dubia and
Pimephales promelas (fathead minnow).

15
C. Length of Chronic Toxicity Tests.
1. Fathead Minnows:
The length of the fathead minnow chronic toxicity test is 7 days.
2. Ceriodaphnia dubia:
The nominal length of the Ceriodaphnia
dubia chronic toxicity test is 7 days. The end
point of the test involves the production of 3 broods by the control organisms. The
test may end after 6 days if 60 percent or more of the controls have had 3 broods and
have produced an average of 15 young per surviving female, as a minimum. The test
may last as long as 8 days if 3 broods have not been produced by the controls. This
could occur if temperatures were to drop, etc.
D. Type of Test.
Chronic toxicity tests will be static renewal tests. Test solutions should be renewed every
24 hours, using one of three sample sets collected throughout the duration of the test. The
first sample should be used to initiate the test on “Day 0" (start of test) and to renew the
solutions at “Day 1" (24 hours). The second sample set should be collected to renew the
test solutions at “Day 2" (48 hours and “Day 3" (72 hours). The third sample should be
collected to renew test solutions at “Day 4" (96 hours), “Day 5" (120 hours) and “Day 6"
(144 hours). Care should be taken not to exceed the allowable holding time for each
sample (see Section 3.E). Ohio EPA prefers the above sample collection arrangement;
however, if plant operation, scheduling difficulties, or other problems arise, an alternative
sample sequence may be used. If an alternative sequence is used, it should be noted on the
test report.
All chronic toxicity tests are definitive tests, designed to quantify the amount of chronic
toxicity. Therefore, a series of dilutions of the effluent shall be tested using a minimum of
five effluent concentrations for each chronic test. The typical dilution series consists of
solutions of 100, 50, 25, 12.5 and 6.25 percent by volume effluent.
There may be instances when it will be advantageous to use a different dilution series.
Selection of the appropriate dilution series is the responsibility of the permittee. Ohio EPA
staff will be available for consultation on the issue of appropriate dilution series. However,
the permittee, in conjunction with the testing laboratory, may exercise professional
judgement on the selection of appropriate dilution series. When selecting a dilution series,
the following two issues should be considered:
1. Allowable Effluent Toxicity:
16
AET is the permissible amount of toxicity for a particular discharge. This value is
determined by factoring the available dilution in the receiving stream with the water
quality criteria for toxicity as well as the effects of any interactive discharges. Should
permit limitations for toxicity be established for a permittee, the AET is the amount
of toxicity that would be allowed. Methods for calculating the AET are contained in
OAC 3745-02-09, as mentioned in Section 2.D.2.a. When selecting a dilution series,
the relationship of that series to the AET should be considered. The series selected
should be one which will yield data to determine if the AET has/has not been
exceeded, yet still identifies the NOEC, LOEC, and IC25.
2. Toxicity in the Effluent:
There may be instances when it is necessary to alter the dilution series in order to
better determine the NOEC, LOEC and IC25. This would be the case in an effluent
that exhibits moderate chronic toxicity. In order to better define the NOEC, LOEC
and IC25, the dilution series has to be shifted to a higher set of dilutions. Also, if
effluent toxicity is consistently exhibited within a certain range, it may be advisable
to adjust the dilution series to focus in that area to better define the NOEC, LOEC
and IC25.
If the chronic toxicity shown by the effluent falls into a large gap in the dilution series
(e.g., between 100 and 50 percent effluent), it may be difficult to discern whether or
not the AET is actually being exceeded. For example, assume a discharge to a stream
has an AET of 1.3 Chronic Toxic Unit (TU
c
) and the dilution series used for testing is
100, 50, 25, 12.5 and 6.25 percent effluent by volume. If the NOEC equals 50
percent and the LOEC equals 100 percent, the TU
c
value equals 1.4 (see Section
3.H.1 for the definition of TU
c
). However, one would be unable to distinguish if the
AET of 1.3 TU
c
was really exceeded. With a dilution series of 100, 75, 50, 25 and
12.5 percent by volume effluent, one would be able to tell if the AET was actually
exceeded. If the LOEC was 100 percent and the NOEC was 75 percent, the AET
would not have been exceeded. However, if the LOEC was 75 percent and the
NOEC was 50 percent, the AET would have been exceeded.
Control and ambient water samples are tested full strength (i.e., no dilutions).
E. Sample Collection.
Effluent samples used to initiate and renew the test solutions in a chronic toxicity test
should be collected as 24-hour composite samples. If the effluent is chlorinated for
disinfection purposes, the effluent sample should be collected at a point prior to
chlorination. The protocols in Section 1.A.1 should be consulted for the handling of a
chlorinated sample if it is impossible to obtain a sample prior to disinfection. However, if
17
dechlorination is an integral part of the disinfection system at the facility, the sample
should be collected at the final outfall.
Unless specifically modified by the NPDES permit, a chronic toxicity test of an effluent
requires that an upstream control sample be collected as well as near-field and far-field
downstream samples during each effluent sampling event. The upstream control sample is
to be collected as a grab sample upstream from the zone of effluent and receiving water
interaction. Care should be taken to assure that any upstream backflow of the effluent is
taken into account when selecting the upstream sampling location.
The near-field downstream sample is to be collected as a grab sample from within the
effluent plume in the immediate vicinity of the outfall. The near-field sample should be
collected in the middle of the effluent plume at a distance of 5 times the water depth at the
point of discharge, down current from the outfall. When water depth at the point of
discharge exceeds 4 feet, the near-field sample should be collected 20 feet (6 meters) down
current from the outfall.
The far-field downstream sample is to be collected as a grab sample within the effluent
plume at a point which represents fairly complete mixing of the effluent and the receiving
water. The available volume of dilution and the mixing characteristics of the receiving
stream influence the selection of an appropriate far-field sampling point. Additional details
are given below:
1. Rapid and Complete Mixing.
In situations where mixing is rapid and complete, the far-field sample should be
collected mid-stream, at a distance of five times the stream width at the point of
discharge, down current from the outfall. Ohio EPA anticipates that rapid and
complete mixing conditions should occur when receiving stream dilution to discharge
ratios are 10:1 or less, and the stream exhibits good pool, run, riffle development. The
presence of rapid and complete mixing should be verified and documented at least
once during the sampling program. If rapid and complete mixing cannot be
documented, sampling should be conducted as if mixing is not rapid and complete.
2. Mixing is not Rapid and Complete
In situations where mixing is not rapid and complete, the effluent plume should be
definitively located during each sampling event. The far-field sample should be
collected mid-plume down current from the outfall at a point five times the stream
width at the point of discharge. When the stream width at the point of discharge
exceeds 500 feet, the far-field sample should be collected mid-plume 2,500 feet (750
meters) down current from the outfall at a maximum. Ohio EPA anticipates that slow
mixing effluents or shore-hugging plumes that persist for considerable distances may
occur when receiving water dilution to discharge ratios are 10:1 or greater, or the
discharge is to a receiving stream that tends to remain in a pooled condition. In these

18
situations, it is imperative that the effluent plume be located and documented for each
sampling event, and that the sample be taken at mid-plume.
The location of the near-field and far-field sampling locations with respect to the effluent
plume must be determined and documented at the time of sampling using temperature
measurements, conductivity measurements, visual observation, a dye study, or other
detailed techniques for delineating the effluent plume. Upon prior approval from Ohio
EPA, an alternative far-field sampling location can be determined from a detailed mixing
zone study.
First use of samples collected for toxicity testing should occur within 36 hours of
completion of sampling.
F. Quality Assurance.
1. Requirements for the Repeating of a Test:
A repeat of a 7-day, short-term chronic toxicity test is mandatory when a combination
of mortality and adverse effects in both laboratory and receiving water controls
exceeds 20 percent for a particular species. A repeat of the test is not necessary if
there is 20 percent or less affected in one of the two control waters.
2. Organism Survival:
The long-term ability of the test organisms to survive can be monitored in a chronic
toxicity test by evaluation of the growth and reproductive success of the control
organisms. Failure of the control organisms to exhibit the following indicators
invalidates the test results and, therefore, a repeat of the test if required.
a. Ceriodaphnia dubia
1. Pretest Culture - Records shall be maintained of the adult Ceriodaphnia
dubia females from which neonates used in the chronic test are to be
obtained. The adult Ceriodaphnia
dubia should be cultured in individual
containers, fed daily, and container solution renewed at least 3 times per
week. If the following conditions are met in the 7-day period prior to
testing, the neonates should be suitable for use:
o
Adult mortality may not exceed 20 percent.
o
A minimum mean number of 20 young per surviving adult in 3 broods
are produced.

19
o
Neonates used in the test are obtained from broods of 8 or more.
2. Controls During Testing - Control organisms shall demonstrate acceptable
survival (see Section 3.F.1). In addition, the controls shall produce an
average of 2.5 broods per test organism with mean production of at least
15 young per surviving animal in the test.
b. Fathead Minnows
Control organisms shall demonstrate acceptable survival (see Section 3.F.1). In
addition, if the larval fish are between 24 and 48 hours old at test initiation, the
controls shall show an average of at least a 3-fold weight increase. If the
fathead minnow larvae are less than 24 hours old at test initiation, the average
dry weight of the control organisms at the end of the test shall equal or exceed
0.250 mg.
3. Dilution Water Substitution:
If a combination of mortality and adverse effects in the upstream control sample
exceeds 20 percent for a particular test organism during a test, or the conditions
specified in Section 3.F.2.a or 3.F.2.b are not fulfilled, an alterative dilution and
control water should be identified and used for subsequent tests. Acceptable
alternative dilution waters may be similar natural waters, rearing unit water,
reconstitute water, or dilute mineral water. The upstream receiving water sample
should still be collected and tested, but the receiving water should not be used as
diluent. Failure to change the dilution water in subsequent tests may result in
invalidation of testing results and a requirement to repeat the test.
4. Number of Organisms:
a. Ceriodaphnia
dubia - 10 organisms is the minimum number that should be used
in each solution of a chronic toxicity test. These organisms should be set up in
10 replicate test chambers for each solution tested (i.e., one per chamber).
b. Fathead Minnows - A minimum of 40 organisms should be used in each
solution of a fathead minnow chronic toxicity test. These organisms should be
set up in a minimum of 3 replicate test chambers for each solution tested.
However, Ohio EPA recommends the use of 4 replicate chambers which results
in a more robust data base.
G. Chemical Analysis.

20
A sufficient volume of effluent shall be collected during each of the 3 composite sampling
periods to allow aliquots to be used in chronic toxicity tests and for chemical analysis. If
no acute effects are seen during the use of the first 2 composite samples for test initiation
and renewal, then only the third aliquot needs to be analyzed for chemical parameters. If
acute effects are documented during the initial or middle stages of the chronic test, then the
aliquot corresponding to the solution causing the acute toxicity shall be analyzed for
chemical parameters. In determining whether an acute effect has occurred in a sample,
Ohio EPA recommends that if mortality and other adverse effects exceed 20 percent, the
testing laboratory consider the issue of acute effects. Ohio EPA feels that this threshold
should be used in conjunction with the professional judgement of the testing laboratory to
determine if an acute effect is being observed.
Bioassay effluent sampling may be coordinated with other permit sampling requirements as
appropriate to avoid duplication. The analyses detailed in the currently effective Part I,
Effluent Limitations and Monitoring Requirements table(s) in the NPDES permit should be
conducted for the effluent sample. In addition, alkalinity and hardness (as CaCO
3
) should
be measured. Chemical analysis must comply with Ohio EPA accepted procedures.
H. Reporting Requirements.
1. Reporting Toxicity Testing Results on Monthly Operating Reports:
NPDES permits require that results of testing be reported on a form acceptable to
Ohio EPA. The results of toxicity tests required under Part I Permit Requirements
shall be reported on EPA Form 4500 (see Attachment 1).
Results for final effluent shall be expressed as toxic units. A chronic toxic unit (TU
c
)
is defined as:
Tu
c
= 100
IC25
For Ceriodaphnia
tests, Tu
c
should also be calculated as:
Tu
c
= 100
square root of (NOEC x LOEC)
Chronic toxic unit values for Ceriodaphnia tests must be calculated based upon the
NOEC x LOEC square root using the most sensitive end point (e.g.,
growth/reproduction or survival) and should be reported. Toxicity test results should
be recorded on the day in which the first sampling event is completed
(i.e., the day
the first composite sample is picked up). If the NOEC equals 100 percent effluent,
the toxicity test result should be reported on Form 4500 as “AA” (below detectable).

21
Form 4500 must be received by Ohio EPA Central Office by the 15th day of the
month following the month in which the toxicity test samples were collected. It may
be necessary to obtain or provide the toxic unit results prior to receipt of the full
laboratory report in order to fulfill this requirement.
Reporting for instream stations shall be in percent of organisms affected. This
percentage should reflect all mortality and atypical behavior or appearance by the test
organisms. Results from upstream stations (those NPDES monitoring stations
numbered as 801, 802, etc.) should be reported as the percent affected from that
sample. In the event that an alternative control/dilution water is used, data from the
upstream ambient station (if required) should still be reported and the fact that an
alternative dilution water was used should be recorded in the comments section on
the form. Downstream stations (those NPDES monitoring stations numbered as 901,
902, etc.) should reflect the results of the far-field sample. If growth or reproduction
are measured as levels that are significantly less than controls at the downstream
station, then the results recorded on the form should indicate that 100 percent of the
organisms were affected, and a note placed in the comment section indicating that it
was a growth or reproductive effect. If no mortality or effects are observed in these
solutions, record “AA” (below detectable) on the 4500 form.
See Attachment 4 for an example of a completed Form 4500 showing the results of a
chronic toxicity test.
Timely submittal of these data allows for input of toxicity testing results into Ohio’s
mainframe computer systems.
2. Information Reported for Chronic Toxicity Tests:
Ohio EPA has established two basic reporting formats for chronic toxicity testing
results, depending upon whether the permittee has effective TU
c
limitations in its
NPDES permit.
a. Reporting for Permittees with Detailed Reporting Requirements - If the
permittee does not have effective toxic unit limitations in its permit, additional
information shall be submitted, as well as
the necessary reporting on Form
4500. Reports containing the additional information for chronic toxicity
monitoring requirements shall be submitted to the following address within 60
days of the initiation of the test:
Permits Section
Ohio EPA-Division of Surface Water
P.O. Box 1049
Columbus, Ohio 43216-1049

22
Reporting of chronic toxicity monitoring requirements shall be submitted on the
“Ohio EPA NPDES Biomonitoring Report Form” for chronic toxicity tests (see
Attachment 5). The biomonitoring report for chronic toxicity tests consists of
the following six parts: 1) General Information; 2) Chronic Toxicity Sampling
Data; 3) Toxicity Test Conditions; 4) Chronic Toxicity Test Results for
Ceriodaphnia dubia; 5) Chronic Toxicity Test Results for Pimephales promelas;
and 6) Additional Toxicity Test Information and Conclusions/Comments. The
following is guidance on completion of the six portions of the biomonitoring
report:
1. General Information - This part of the form is applicable to both the acute
and the chronic tests. Much of the information in this part is self-
explanatory. However, additional details are given in Section 2.H.2.a.1.
2. Chronic Toxicity Test Sampling Data - Describe conditions associated
with the collection of samples. This part should be filled out for each
outfall that is tested. The information entered in this part of the form is
self-explanatory.
3. Toxicity Test Conditions - Describe the conditions during the toxicity test.
This part should be filled out for each species tested. Additional details
describing some of the information requested are listed below:
o
For Test Type and Duration, the test type would typically be static
renewal; durations should be listed in days.
o
The Renewal of Test Solutions lists the collection date (MM/DD) for the
sample that was used for each daily renewal.
o
For Dilution and Primary Control Water, list the source of the dilution
and primary control water (e.g., receiving water, rearing unit water,
dilute mineral water, etc.).
o
The Secondary Control Water lists the source of the secondary control
water (e.g., hard reconstituted water, receiving water, dilute mineral
water, etc.).
o
For aeration, indicate whether or not the sample required aeration and if
aeration was required before or during the test.
4. Chronic Toxicity Test Results - Describe the results of the Ceriodaphnia
dubia survival and reproduction test. Much of the information that is

23
requested is self-explanatory. Additional details describing some of the
information requested are listed below:
o
Cumulative Percent Mortality is the cumulative total of dead organisms,
expressed as a percentage of the total test organisms for that solution
recorded every 24 hours. The result should be recorded on the line
corresponding to the solution that was tested.
o
Cumulative Percent Affected is the cumulative total of organisms
showing some adverse effect (e.g., death, disorientation, atypical
appearance or behavior), expressed as a percentage of the total test
organisms for that solution recorded every 24 hours. The result should
be recorded in the parentheses below the line showing percent mortality
at the time for that solution.
o
Number of Young Produced is the total number of young produced by
all Ceriodaphnia dubia for a particular solution (report in the Total
column) and the mean number of young per surviving organism (report
in the Mean column). An animal that dies before producing young, if it
has not been identified as a male, is included in the analyses with zero
entered as the number of young produced. Those solutions showing
significantly less reproduction than the primary control (assuming all
Quality Assurance parameters are met - see Section 3.F) should be
designated with an asterisk.
o
For Method(s) Used to Determine Values, list the graphical or statistical
methods used to derive the LC
50
, NOEC, LOEC, and IC25. For the
NOEC, LOEC and IC25, the method used to determine a significant
difference between the test solution and the primary control should be
listed.
o
Chronic Value for Mortality is the geometric mean of the LOEC and
NOEC for mortality. It is calculated as the square root of (NOEC x
LOEC).
o
Chronic Value for Reproduction is the geometric mean of the LOEC and
NOEC for reproduction. It is calculated as the square root of (NOEC x
LOEC).
5. Chronic Toxicity Test Results for Pimephales promelas - Describe the
results of the Pimephales
promelas survival and growth test. Much of this
information is self-explanatory or is described under the results for the
Ceriodaphnia
dubia survival and reproduction test, except that dry weights
24
based on the original number of larvae in each replicate should be used
instead of number of young, and a chronic value is calculated for growth
rather than reproduction.
6. Additional Toxicity Test Information - This portion is used to present
information about the effluent plume during sampling, as well as any
conclusions that may be drawn from the data, and should be completed for
each species and outfall. Additional details on the information requested
are listed below:
o
For Method(s) Used to Verify Near-Field and/or Far-Field Sampling
Locations ..., complete this portion indicating the method used to verify
the location of the effluent plume during the sampling (e.g.,
conductivity, temperature, visual, etc.).
o
Conclusions/Comments should be completed for each species and
outfall tested as the permittee or testing laboratory as deemed
appropriate. The information listed under Additional Toxicity Test
information must be attached to the report.
b. Reporting for Permittees with Toxic Unit Limits - If the permittee has effective
toxic unit permit limitations, the only reporting required is on Form 4500,
provided that the SOP requirements (see Section 1.B) has been fulfilled. All
other data elements required by this Section (see items 1 through 25, below)
shall be retained by the permittee in accordance with Part III.7, Records
Retention, of the NPDES permit. Although records retention requirements
specify that records be maintained for 3 years, Ohio EPA recommends that
toxicity test information be retained for 5 years (or through the subsequent
permit renewal).
The following information is required to be retained as supporting information
for each test. This data will be used, if necessary, to assure the validity of data
submitted on Form 4500.
1. Name and address of the testing laboratory.
2. Name and address of the facility which is the source of the effluent tested.
3. Ohio EPA and NPDES permit numbers for the facility.
4. Receiving water tested and/or used.
5. Source of dilution water.

25
6. Date and time of sample collection.
7. Collector(s) name(s).
8. Type of toxicity test.
9. Test organisms used.
10. Test organism origin and acclimation process.
11. Number of organisms per container and per concentration.
12. Test container size, number per concentration, and depth of test solution.
13. Concentrations tested and volume.
14. Test temperature.
15. Results of chemical analyses.
16. Results of physicochemical measurements taken during the test.
17. Definition of adverse effects measured in the test (end points).
18. Number of organisms in each concentration showing the adverse effects at
specified times.
19. Median lethal concentrations (LC
50
) and/or the median effect
concentrations (EC
50
) for each 24-hour interval during the test, confidence
limits for those values, and the methods used for the calculations.
20. Exact details for the near-field and far-field sample locations in relation to
the outfall and plume location along with the results from temperature,
conductivity or dye study used to confirm the effluent plume. If
conducted, results of the detailed mixing zone study shall be provided.
21. All raw data obtained in the toxicity test concerning organism survival,
growth of fathead minnow larvae, and reproduction of Ceriodaphnia
dubia. The raw data shall be in tabular form according to date and effluent
concentrations and/or receiving water locations.
22. The LOEC and the NOEC for survival and reproduction of Ceriodaphnia
dubia.

26
23. The IC25 values for reduction in fathead minnow growth and
Ceriodaphnia dubia reproduction.
24. The IC50 values for reduction in fathead minnow growth and
Ceriodaphnia dubia reproduction.
25. The average daily discharge of the effluent(s) of concern and the receiving
water during the bioassay sampling.
26. Any other relevant information.
Section 4: Requirements for Instream Biosurveys
This Section discusses the details of NPDES permit requirements for instream biological
community assessment designed to indicate the attainment or nonattainment of the biological
criteria (biocriteria) specified in the Ohio WQS, OAC 3745-1-07.
A. Function of Instream Biological Surveys.
Ohio EPA has established biocriteria which specify the expected performance of
indigenous fish and macroinvertebrate communities in waters of the state. These
expectations are based upon the division of the state into five ecoregions having similar
physical and biological characteristics, and the establishment of biocriteria based upon
least-impacted reference sites within each ecoregion. Direct measurement of the instream
biological communities can be used to define the effect of a discharge upon the receiving
water and to demonstrate attainment or nonattainment of the applicable biocriteria in the
WQS. Biological criteria reflect the cumulative effect of physical, chemical and biological
impacts in the aquatic environment and an end result of our efforts to improve water
resource integrity via regulatory programs.
B. Factors Examined in Conducting a Biosurvey.
There are three primary factors assessed when conducting a biosurvey. The factors are the
structure and function of the fish community, the structure and function of the
macroinvertebrate community and the quality of the habitat.
1. Fish Community Structure and Function:
Fish community structure is typically evaluated using the Modified Index of Well
Being (MIwb). This index uses numbers of species and individuals, as well as
biomass, to characterize the structure of the community. Ohio EPA has refined this
index to make it more sensitive to a variety of community disturbances by removing
12 species of tolerant fish, hybrids and exotics from the numbers and biomass
27
calculations, while retaining them in the calculations of the diversity indices used to
calculate the MIwb.
Fish community structure and function are typically examined using the Index of
Biotic Integrity (IBI). This index uses the proportions of the fish community that
reflect various functional roles in the aquatic environment to rank the performance of
a site. Twelve (12) of these values (metrics0 are scored and summed, with the total
score representing the IBI. This index has proven to be a good indicator of fish
community disturbances, or lack thereof.
2. Macroinvertebrate Community Structure and Function:
Macroinvertebrate community health is evaluated using the Invertebrate Community
Index (ICI). This index was developed along the same lines as the IBI and provides
an accurate reflection of disturbances to aquatic macroinvertebrates.
3. Habitat Quality:
The quality of the habitat at each fish sampling station is evaluated using the QHEI.
This index provides a relative scale for defining habitat quality and is used to assist in
judging the potential aquatic life uses of a water body, as well as to influence
judgements made pertaining to causes and sources of nonattainment of the biocriteria.
C. Time Period for Performing Survey Work.
Biosurvey sampling should be conducted during the period of June 15 through October 15
and follow the attached guidelines (Attachment 6). Sampling done outside of this time
frame may result in invalidation of the results for comparison with the WQS. If sampling
must be conducted outside of this time frame, prior approval must be obtained from Ohio
EPA.
D. Type of Survey.
An instream community survey conducted to provide comparison with the biocriteria
should consist of both fish and macroinvertebrate sampling unless otherwise specified by
Ohio EPA. This will allow for complete definition of any problems, or lack of problems,
due to the broad range of organism sensitivities that will be measured.
E. Sample Collection.
Instream biological samples should be collected strictly in accordance with the protocols
specified in Section 1.A.2 of this guidance. Deviation from these protocols may only be
done with prior approval from Ohio EPA. Chemical sampling must be done in accordance

28
with Section 4.G. Field Monitoring Guidelines and guidelines for locating sample sites are
included as Attachment 6.
F. Study Plan Submission.
Prior to the initiation of an instream biomonitoring program, the permittee shall submit a
study plan detailing the methods, sampling sites and proposed sampling times for the study
to the address listed in Section 3.H.2.a. In addition, a pre-field meeting may be requested
by Ohio EPA for the purposes of coordinating standard methods and answering questions.
The permittee should not begin sampling until approval of the study plan has been granted
by Ohio EPA.
G. Sampling of Ambient Waters for Instream Biosurveys.
Chemical analysis of ambient waters must be performed in conjunction with an instream
biological survey. Chemical sampling must be conducted at least 6 times at each site
between June 15 and October 15 at intervals not to exceed 2 weeks nor less than 1 week.
Sediment samples should be collected once at each site in October.
Parameters analyzed at each site should be relevant to the NPDES permit monitoring
requirements and any interactive impacts, including nonpoint sources, that occur in the
study area.
H. Reporting Requirements.
If a permittee, consulting firm, or contract laboratory has conducted a stream survey to
assess attainment of biological WQS criteria, a final report should be submitted which
contains the following information:
1. Name of facility.
2. The receiving water of the discharge and the subsequent stream network.
3. A description of the facility, including the processes used at the facility, a description
of any treatment facilities, the physical location of the facility in relation to the
receiving water, and any other items unique to that facility. A diagram of the facility
showing relevant outfalls should be included.
4. A characterization of the effluent from the facility in terms of any chemical or
biological testing that has been performed.
5. Descriptions of all sampling sites in the study area, including a description of the
location of the site, rationale for site selection, length of the sampling zone in meters,
29
the nature of the habitat at the site, the location in the stream (using the River Mile
Index), and any other factors unique to the sampling sites.
6. A listing of the name and model number of all sampling equipment used in the
collection of the survey data.
7. Descriptions of all electrofishing configurations used in the survey.
8. Types of boats (if any) used in the survey.
9. A description of exact methods for demarcation of the sampling zone, including
descriptions of landmarks and other marks used to define sampling sites.
10. A diagram of the course followed as each sampling zone was traversed on each
sampling date.
11. A description of sampling preservation methods.
12. A listing of all taxonomic keys utilized for specimen identification.
13. The location of the reference collection used to verify difficult-to-identify specimens
and any other sources used to verify identifications.
14. The exact methods used to construct the Hester-Dendy samplers or the source of
purchase.
15. Methods used for anchoring Hester-Dendy samplers.
16. A description of the methods used to identify Dipterans of the family Chironomidae.
17. Copies of all raw data sheets.
18. A description of the methods used to calculate the QHEI, the IBI, the MIwb and the
ICI for each site.
19. A description of qualitative macroinvertebrates sampling techniques.
20. A complete description of any statistical analysis that was performed on the data.
21. Date(s) and time(s) of sampling. This should include the amount of time spent
electrofishing (in seconds0 on each sampling site for each date.

30
22. Results of the stream survey, in terms of species presence, absence and relative
numbers for each study site.
23. A discussion of historic data pertaining to the locality of the study sites or that stream
segment.
24. The calculated IBI, MIwb and the ICI used for comparison with the biological water
quality criteria.
25. Raw data submitted in computer format for entry into the Ohio EPA Fish Information
System (FINS) and Macroinvertebrate Data Gathering and Evaluation System
(MIDGES). This includes QHEI sheets.
26. The biological criteria used for comparison with the stream sampling data and the
rationale behind the selection of the criteria.
27. The calculated QHEI values.
28. A discussion of the study results in terms of impacts from the facility in question and
other facilities that may have been studied.
29. Any other relevant information.
I. Collection Permit.
In order to conduct instream biological surveys, it is necessary to secure a permit for the
collection of aquatic animals for scientific purposes. In order to obtain a permit, the Ohio
Department of Natural Resources (ODNR) should be contacted at the following address:
ODNR-Division of Wildlife
License & Permit Section
1840 Belcher Drive
Columbus, Ohio 43224
(telephone: 614-265-7040)
Section 5: NPDES Permit Application Requirements
All publicly-owned treatment works (POTW’s) with a design flow greater than 1 million gallons
per day (1 MGD), and all POTW’s with approved pretreatment programs, must submit the
results of whole effluent toxicity (WET) testing with their application for renewal of their
NPDES permit as required by 40 CFR 122.21(j).

31
A. Guidelines for Submittal of This Data:
1. The data must have been collected since the last permit action (e.g., renewal or
modification) or any major modifications to the POTW.
2. The data must reflect current operations at the POTW.
3. WET data collected by Ohio EPA is acceptable for this submission.
4. The minimum WET testing requirement is an acute screening test, as described in
Section 2.D.1.a., for both fathead minnows and Ceriodaphnia dubia.
5. If the results of the acute screen test show greater than 50 percent mortality in 100
percent effluent, a definitive acute toxicity test, as described in Section 2.D.2, shall be
conducted for the organism showing the toxicity, and the results submitted with the
application.
6. At the permittee’s option, the acute screening test may be waived and only the acute
definitive test conducted.
7. The results of the toxicity test(s) shall be submitted with the permit application. The
permit application is incomplete until receipt of this data.
ATTACHMENT 1
Form 4500

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
SAMPLING STATION DESCRIPTION
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
09
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER
ATTACHMENT 2
Example Form 4500 Showing Acute Toxicity Test Results

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023001 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
001 Final Effluent
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
22
24 24
Tox-Un
AC-cer
TUA
REPORTING CODE
61425
Tox-Un
ACU-Pi
TUA
REPORTING CODE
61427
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
1.8 0.8
09
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023801 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
801 Upstream
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
48 H AC
C. Dubi
% Eff
REPORTING CODE
61432
96 H AC
Pimeph
% Eff
REPORTING CODE
61435
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
AA AA
09
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023901 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
901 Downstream
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
48 H AC
C. Dubi
% Eff
REPORTING CODE
61432
96 H AC
Pimeph
% Eff
REPORTING CODE
61435
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
AA 10
09
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER

Sample Data Set
Acute Toxicity Test Data
Anytown WWTP
Fathead Minnow Ceriodaphnia dubia
Concentration % Mortality % Affected % Mortality % Affected
100 35 40 100 100
50 0 0 25 35
25 0 0 0 0
12.5 0 0 0 0
6.25 0 0 0 0
Upstream Control 00 0 0
Downstream Sample 10 10 0 0
LC
50
>100 59.9
EC
50
>100 55.8
Tu
a
0.8 1.8
ATTACHMENT 3
Ohio EPA NPDES Biomonitoring Report Form Acute Toxicity Test

Date Created: 5/24/91 Page ____ of ____
Last Revised: 8/10/98
OHIO EPA NPDES BIOMONITORING REPORT FORM
GENERAL INFORMATION
1. Facility Name: Reporting Date:
2. Address:
3. Ohio EPA Permit Number: 4. Application (NPDES) No.
5. Facility Contact: 6. Phone No.: ( )
7. Consultant/Testing Lab Name:
8. Consultant/Lab Contact: 9. Phone No.: ( )
10. Receiving Water(s) of Discharge:
11. Outfall(s) Tested :
Average Daily Flows :
on Day Sampled (MGD)
12. Is your current Standard Operating Procedure (SOP) Manual on file with Ohio EPA?
(Yes/No) . If yes, date submitted: . If no, a SOP that
follows Ohio EPA and/or U.S. EPA protocols must be submitted as soon as possible
in order to eliminate the need to include this information with every report.
I certify under penality of law that I have personally examined and am familiar
with the information submitted in this document and based on my inquiry of those
individuals immediately responsible for obtaining the information, I believe the
submitted information is true, accurate and complete. I am aware that there are
significant penalties for submitting false information, including the possibility
of fine and imprisonment.
Signature Date
Name (typed or printed) Title

OEPA Permit No.: Page ____ of ____
ACUTE TOXICITY TEST SAMPLING DATA
TABLE
Sampling Summary for Acute Toxicity Tests
Sampling Location & Description
Sample Collection
Beginning Ending
MM/DD/Time MM/DD/Time
Weather/Receiving
Stream Conditions
Final Effluent:
Outfall No.:
Type (Grab/Composite):
Volume Collected:
Upstream Station:
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Downstream Station (Near-field):
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Additional Stations (If needed):
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:

OEPA Permit No.: Page ____ of ____
TOXICITY TEST CONDITIONS
TABLE
Summary of Toxicity Test Conditions
1. Test Species and Age:
2. Test Type and Duration:
3. Test Dates:
4. Test Temperature (
o
C):
5. Light Quality:
6. Photoperiod:
7. Feeding Regime:
8. Size of Test Vessel:
9. Volume and Depth of
Test Solutions
10. No. of Test Organisms
per Test Vessel:
11. No. of Test Vessels
per Test Solution:
12. Total No. of Test Organisms
per Test Solution:
13. Test Concentrations (as
percent by volume effluent):
14. Renewal of Test Solutions:
15. Dilution and Primary
Control Water:
16. Secondary Control Water:
17. Aeration? Before/During Test:
18. Endpoints Measures:
19. If secondary control water used as
diluent due to toxicity in primary
control water, indicate number of
consecutive tests conducted with
alternative diluent:

OEPA Permit No.: Page ____ of ____
ACUTE TOXICITY TEST RESULTS
TABLE
Results of a -Hour Static Acute Toxicity Test
(genus) (species)
Conducted - Using Effluent from Outfall .
(mm/dd/yy) (mm/dd/yy) (number)
Test Solutions
Cumulative Percent Mortality
(Cumulative Percent Affected)
a
24-Hr 48-Hr 72-Hr 96-Hr
LC50 Values
(EC50%) Values)
24-Hr 48-Hr 72-Hr 96-Hr
Primary Control/
Dilution water
( ) ( ) ( ) ( )
( ) ( ) ( ) ( )
Secondary
Control
( ) ( ) ( ) ( )
% Effluent
( ) ( ) ( ) ( )
LC50 95% Confidence Limits
(EC50 95% Confidence Limits)
24-Hr 48-Hr 72-Hr 96-Hr
% Effluent
( ) ( ) ( ) ( )
% Effluent
( ) ( ) ( ) ( )
LL
UL
LL ( ) ( ) ( ) ( )
UL (
) ( ) ( ) ( )
LL = Lower Limit
UL = Upper Limit
% Effluent
( ) ( ) ( ) ( )
100 % Effluent
( ) ( ) ( ) ( )
Near-Field
Sample
( ) ( ) ( ) ( )
Calculate TUa Value:
Method(s) Used to Determine LC50,
EC50 and Confidence Limit Values:
a - cumulative percent affected is the total percentage of test organisms observed dead,
immotile, exhibiting loss of equilibrium or other defined endpoints (specify below)
.

OEPA Permit No.: Page ____ of ____
ADDITIONAL TOXICITY TEST INFORMATION
1. Submit all raw data and statistical calculations/printouts obtained during the test(s).
Data must be presented in tabular form and must include all physical and/or chemical
measurements recorded during the tests and sampling. (E.g. temperature,
conductivity, dissolved oxygen, pH, hardness, alkalinity, etc.).
2. Method(s) used to verify near-field and/or far-field sampling locations must be
included if instream testing is required. Maps, sketches and/or drawings may be used
to show locations.
CONCLUSIONS/COMMENTS
Indicate below any other relevant information that may aid in the evaluation of this
report. Include any deviations from your SOP that were necessary for these tests and
any recent Standard Reference Toxicant (SRT) results obtained. Do these results
agree with previous SRT results? Attach additional pages as needed.
ATTACHMENT 4
Example Form 4500 Showing Chronic Toxicity Test Results

Sample Data Set
Chronic Toxicity Test Data
Anytown WWTP
Fathead Minnow Ceriodaphnia dubia
Concentration Percent Mortality Percent Mortality
100 100* 20 **
50 45* 30 **
25 10 10 **
12.5 20 0
6.25 7.5 0
Upstream Control 2.5 0
Lab Control 2.5 0
Near-field Sample 95* 0 **
Far-field Sample 5.0 0
Survival Growth Survival Reproduction
NOEC 25% 25% 100 12.5%
LOEC 50% 50% >100 25.0%
Tu
c
2.8 2.8 <1.0 5.7
IC
25
33.4% 21.7%
IC
50
48.3% 53.6%
Tu
c
3.0 4.6
NOTE: The 100% effluent concerntration
Showed no significant difference from the
Upstream control by Fisher’s Exact Test.
* Denotes significant difference in survival from the upstream control (P=0.05).
** Denotes significant difference in growth and/or reproduction upstream control (P=0.05).

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023801 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
801 Upstream
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
7 DAY C
C. Dubi
% Eff
REPORTING CODE
61438
7 DAY C
Pimeph
% Eff
REPORTING CODE
61441
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
09
10
11
12
13
14
AA 2.5
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023001 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
001 Final Effluent
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
Tox-Un
CHR-CE
TUC
REPORTING CODE
61426
Tox-Un
CHR-PI
TUC
REPORTING CODE
61428
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
09
10
11
12
13
14
4.6 3.0
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER
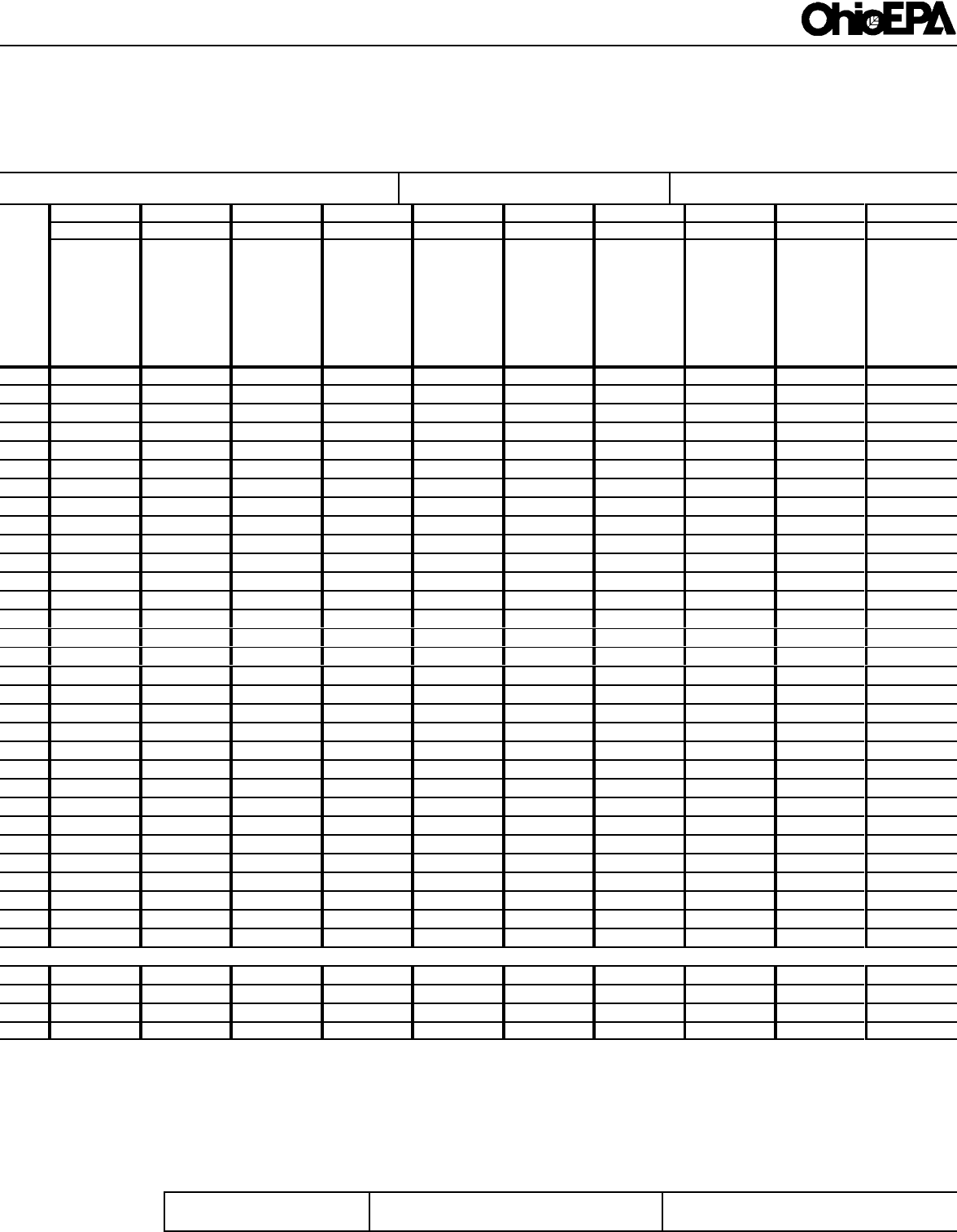
MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE PRINTING DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023901 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
901 Near field
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
7 Day C
C. Dubi
% Eff
REPORTING CODE
61438
7 Day C
Pimeph
% Eff
REPORTING CODE
61441
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
09
10
11
12
13
14
100 100
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
100% Affected in near field for fathead minnows is a result of a significant mortality effect.
100% Affected in near field for Ceriodaphnia is a result of a significant reproduction effect.
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER

MONTHLY REPORT FORM 4500 REPORTED
NAME, ADDRESS, CITY, COUNTY, ZIP STATION CODE DATE (MONTH, YEAR) PAGE
PRINTI
NG DATE APPLICATION NO.
OF
Anytown WWTP 2PQ00023902 Oct. 1991 1 1
238 West End Road
Anytown Fair Plum SAMPLING STATION DESCRIPTION
43026
902 Far field Stream
NOTE: THIS FORM MUST BE TYPED
IN(1) - ENTER 1 FOR CONTINUOUS, 2 FOR COMPOSITE, 3 FOR GRAB SAMPLE
IN(2) - ENTER FREQUENCY OF SAMPLING
REPORTING LAB ANALYST
ENTER ANALYSIS PERFORMED
AND CODE NO. AT RIGHT
(1)
(2)
DAY
33
11
7 DAY C
C. Dubi
% Eff
REPORTING CODE
61438
7 DAY C
Pimeph
% Eff
REPORTING CODE
61441
REPORTING CODE
REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE REPORTING CODE
01
02
03
04
05
06
07
08
09
10
11
12
13
14
AA 3
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
TOTAL
AVG.
MAX.
MIN.
ADDITIONAL REMARKS (AH REPORTING CODES MUST BE EXPLAINED IN THIS SECTION)
DISTRIBUTION
WHITE - AGENCY
GREEN - REPORTER
I CERTIFY UNDER THE PENALTY OF LAW THAT I HAVE PERSONALLY EXAMINED AND AM FAMILIAR WITH THE INFORMATION SUBMITTED AND BASED ON MY INQUIRY OF
THOSE INDIVIDUALS IMMEDIATELY RESPONSIBLE FOR OBTAINING THE INFORMATION, I BELIEVE THE SUBMITTED INFORMATION IS TRUE, ACCURATE AND COMPLETE.
I AM AWARE THAT THERE ARE SIGNIFICANT PENALTIES FOR SUBMITTING FALSE INFORMATION, INCLUDING THE POSSIBILITY OF FINE AND IMPRISONMENT.
FORM NO EPA 4500 (8-91)
FORMERLY EPA SUR1
DATE REPORT COMPLETED SIGNATURE OF REPORTER TITLE OF REPORTER
ATTACHMENT 5
Ohio EPA NPDES Biomonitoring Report Form Chronic Toxicity Test

Date Created: 5/24/91 Page ____ of ____
Last Revised: 8/10/98
OHIO EPA NPDES BIOMONITORING REPORT FORM
GENERAL INFORMATION
1. Facility Name: Reporting Date:
2. Address:
3. Ohio EPA Permit Number: 4. Application (NPDES) No.
5. Facility Contact: 6. Phone No.: ( )
7. Consultant/Testing Lab Name:
8. Consultant/Lab Contact: 9. Phone No.: ( )
10. Receiving Water(s) of Discharge:
11. Outfall(s) Tested :
Average Daily Flows :
on Day Sampled (MGD)
12. Is your current Standard Operating Procedure (SOP) Manual on file with Ohio EPA?
(Yes/No) . If yes, date submitted: . If no, a SOP that
follows Ohio EPA and/or U.S. EPA protocols must be submitted as soon as possible
in order to eliminate the need to include this information with every report.
I certify under penality of law that I have personally examined and am familiar
with the information submitted in this document and based on my inquiry of those
individuals immediately responsible for obtaining the information, I believe the
submitted information is true, accurate and complete. I am aware that there are
significant penalties for submitting false information, including the possibility
of fine and imprisonment.
Signature Date
Name (typed or printed) Title

OEPA Permit No.: Page of
CHRONIC TOXICITY TEST RESULTS FOR FATHEAD MINNOWS (Pimephales
promelas)
TABLE
Test Solutions Cumulative Percent Mortality
a
(Cumulative Percent Adversely Affected)
a
Test Day
Mean Dry Weight
a
Day 1 2 3 4 5 6 7 Mean SD
Primary control/
Upstream diluent
Secondary control
(Rearing Unit Water)
6.25%
12.5%
25%
50%
100%
Near-field
Far-field
LC
50
Values (%): Calculated TU
c
Value for
Survival:
95 Percent
Confidence Limits
LL
UL
EC
50
Values (%): >100 >100 >100 >100 >100 >100 >100 Calculated TU
c
Value for
Growth:
95 Percent
Confidence Limits
LL
UL
7-day NOEC for Mortality: 7-day NOEC for Growth: Method(s) Used to
Determine Values:
Mortality
Growth
7-day LOEC for Mortality: 7-day LOEC for Growth:
Chronic Value for Mortality: Chronic Value for Growth:
IC25: IC50:
a
- indicate significant difference from the primary control with an * (p < 0.05)
OEPA Permit No.: Page of
CHRONIC TOXICITY TEST RESULTS FOR Ceriodaphnia
dubia
TABLE
Test Solutions Cumulative Percent Mortality
a
(Cumulative Percent Adversely Affected)
a
Test Day
Number of Young
Produced
a
1 2 3 4 5 6 7 Total Mean
Primary control/
Upstream diluent
Secondary Control (Hard
Reconstituted Water)
6.25%
12.5%
25.0%
50.0%
100%
Far-field
Near-field
LC
50
Values (%): Calculated TU
c
Value for
Survival:
95 Percent
Confidence Limits
LL
UL
EC
50
Values (%): Calculated TU
c
Value for
Reproduction:
95 Percent
Confidence Limits
LL
UL
7-day NOEC for Mortality: 7-day NOEC for Reproduction: Method(s) Used to
Determine Values:
Mortality
Reproduction
7-day LOEC for Mortality: 7-day LOEC for Reproduction:
Chronic Value for Mortality: Chronic Value for Reproduction:
IC25: IC50:
a - indicate significant difference from the primary control with an * (p < 0.05)

OEPA Permit No.: Page ____ of ____
CHRONIC TOXICITY TEST SAMPLING DATA
TABLE
Sampling Summary for Chronic Toxicity Tests
Sampling Location & Description Sample
Sample Collection
Beginning Ending
MM/DD/Time MM/DD/Time
Weather/Receiving
Stream Conditions
Final Effluent:
Outfall No.:
Type (Grab/Composite):
Volume Collected:
Upstream Station:
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Downstream Station (Near-field):
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Downstream Station (Far-field):
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Additional Stations (If needed):
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
Waterbody:
Station No.:
Type (Grab/Composite):
Volume Collected:
1st
2nd
3rd
1st
2nd
3rd
1st
2nd
3rd
1st
2nd
3rd
1st
2nd
3rd
1st
2nd
3rd

OEPA Permit No.: Page ____ of ____
TOXICITY TEST CONDITIONS
TABLE
Summary of Toxicity Test Conditions
1. Test Species and Age:
2. Test Type and Duration:
3. Test Dates:
4. Test Temperature (
o
C):
5. Light Quality:
6. Photoperiod:
7. Feeding Regime:
8. Size of Test Vessel:
9. Volume and Depth of
Test Solutions
10. No. of Test Organisms
per Test Vessel:
11. No. of Test Vessels
per Test Solution:
12. Total No. of Test Organisms
per Test Solution:
13. Test Concentrations (as
percent by volume effluent):
14. Renewal of Test Solutions:
15. Dilution and Primary
Control Water:
16. Secondary Control Water:
17. Aeration? Before/During Test:
18. Endpoints Measures:
19. If secondary control water used as
diluent due to toxicity in primary
control water, indicate number of
consecutive tests conducted with
alternative diluent:

OEPA Permit No.: Page ____ of ____
ADDITIONAL TOXICITY TEST INFORMATION
1. Submit all raw data and statistical calculations/printouts obtained during the test(s).
Data must be presented in tabular form and must include all physical and/or
chemical measurements recorded during the tests and sampling. (E.g. temperature,
conductivity, dissolved oxygen, pH, hardness, alkalinity, etc.).
2. Method(s) used to verify near-field and/or far-field sampling locations must be
included if instream testing is required. Maps, sketches and/or drawings may be
used to show locations.
CONCLUSIONS/COMMENTS
Indicate below any other relevant information that may aid in the evaluation of this
report. Include any deviations from your SOP that were necessary for these tests and
any recent Standard Reference Toxicant (SRT) results obtained. Do these results
agree with previous SRT results? Attach additional pages as needed.
ATTACHMENT 6
Biosurvey Sampling Information

WQP&A-EAS Field Monitoring Guidelines January 1990
FIELD MONITORING GUIDELINES FOR
CHEMICAL AND BIOLOGICAL ASSESSMENTS
Ohio EPA, Division of Water Quality Planning & Assessment
Ecological Assessment Section
Chemical Analysis
• Chemical sampling should be conducted at least 6 times at each site between
June 15 and October 15 at intervals not to exceed two weeks nor less than one
week. Sediment samples should be collected once at each site in October.
• Parameter coverage should be relevant to the NPDES permit monitoring
requirements and any interactive impacts, including nonpoint sources, that occur
in the study area.
Biological Sampling
• Both fish and macroinvertebrate communities should be evaluated, unless
specified otherwise by Ohio EPA-WQP&A.
• Macroinvertebrate sampling is conducted using modified Hester-Dendy artificial
substrate samplers (cluster of 5 plates) set for a 6-week colonization period at
each site, between July 1 and September 30. This sample is to be supplemented
with a qualitative, dip-net/handpick sample of the natural substrate at the time
the artificial substrates are retrieved.
• Fish sampling is conducted using pulsed D.C. electrofishing gear exclusively.
Sampling effort is determined by distance, although a minimum sampling time
is specified for method. Each site is to be sampled two or three times (one pass
may be permitted by Ohio EPA in certain situations). All sampling should be
conducted between June 15 and October 15.
• Specific field and laboratory procedures should conform to those outlined in
Biological Criteria for the Protection of Aquatic Life: Volumes II and III
. These
are periodically updated and users should be aware of the latest version of these
documents.
Aquatic Life Use Attainment
• Attainment of aquatic life uses is determined using biocriteria outlined in the
Biological Criteria for the Protection of Aquatic Life: Volume II
.

Effluent location
Area of Initial Mixing
(AIM) - usually a few meters
in length and width.
Sampling site begins at dst.
boundary of AIM. Fish zone
is 50m for wading/headwater
sites and 100m for boat sites.
Artificial substrates go
here.
The next “regulation” sampling site
should be within 0.5 mi. of the outfall
taking available habitat into account.
Mixing Zone Sampling Design for Biosurveys
The purpose of Mixing Zone sites is to determine if any unusual
avoidance of the mixing zone area is evident or if any other evidence
of acute toxicity is apparent in the indigenous biological communities.
These results are not compared to the biocriteria in the Ohio WQS.
