
CHEM 1010 EXPERIMENT 1
1
* Instructions: Please complete the following fields, corresponding to Experiment 1 in Chemistry
1010H. If you have any questions, please contact the laboratory coordinator.*
Name: Esther Schachter Student ID number: 0731405
Experiment Date: 24/01/2022
CHEM1010H – LAB REPORT REMOTE EXPERIMENT 1 – Calorimetry
Question 1: Complete the following Tables using the data provided for your lab section.
PART 1: Heat Capacity of the Calorimeter
Mass of weighing bottle + paper towel + ice (g): 29.9847
Mass of weighing bottle + wet paper towel (g): 21.6281
Table 1: Temperature over time measurements to determine the heat capacity of the calorimeter
Time
(min.)
Temperature
(°C)
Time (min.)
Temperature
(°C)
Time
(min.)
Temperature
(°C)
E1 = 0.0
17.4
7.0
10.2
14.0
11.2
E2 = 1.0
17.4
8.0
10.3
15.0
11.4
E3 = 2.0
17.4
9.0
10.3
3.0
9.8
10.0
10.5
4.0
9.8
11.0
10.8
5.0
10.1
12.0
10.9
6.0
10.2
13.0
11.1

CHEM 1010 EXPERIMENT 1
2
PART 2: Heat Reaction for Mg and HCl
Mass of Weighing paper + Mg (g): 0.4018
Mass of Weighing paper after transferred (g): 0.2153
Table 2: Temperature and time measurements to determine the heat of reaction of Magnesium and
Hydrochloric Acid
Time
(min.)
Temperature
(°C)
Time (min.)
Temperature
(°C)
Time
(min.)
Temperature
(°C)
E1 = 0.0
17.5
6.0
27.2
12.0
26.7
E2 = 1.0
17.5
7.0
27.1
13.0
26.8
E3 = 2.0
17.5
8.0
27.0
14.0
26.5
3.0
25.4
9.0
26.9
15.0
26.3
4.0
26.5
10.0
26.7
5.0
27.2
11.0
26.6
PART 3: Heat Neutralization of a Strong Acid and Strong Base
Table 3: Temperature and time measurements to determine the heat of neutralization of a strong
acid (HCl) and a strong base (NaOH)
Time
(min.)
Temperature
(°C)
Time (min.)
Temperature
(°C)
Time
(min.)
Temperature
(°C)
E1 = 0.0
17.6
6.0
24.5
12.0
23.5
E2 = 1.0
17.6
7.0
24.4
E3 = 2.0
17.6
8.0
24.3
3.0
24.9
9.0
24.1
4.0
24.9
10.0
23.8
5.0
24.7
11.0
23.7

CHEM 1010 EXPERIMENT 1
3
PART 4: Heat Solution of NH
4
NO
3
Mass of Weighing paper + NH
4
NO
3
(g): 1.2199
Mass of Weighing paper after transferred (g): 0.2172
Table 4: Temperature and time measurements to determine the heat of solution for NH
4
NO
3
in
water
Time
(min.)
Temperature
(°C)
Time (min.)
Temperature
(°C)
Time
(min.)
Temperature
(°C)
E1 = 0.0
17.4
6.0
14.5
12.0
15.2
E2 = 1.0
17.4
7.0
14.6
E3 = 2.0
17.4
8.0
14.6
3.0
14.1
9.0
14.8
4.0
14.1
10.0
14.9
5.0
14.3
11.0
15.0
Question 2: What assumptions, with regard to the conditions of each of the three reaction
procedures (Part 2-4), must be made to determine the required enthalpy values (∆H
rxn
)
? i.e.
why can you use equation (7) rather than equation (6)?
ANSWER:
We would use equation (7) rather than equation (6) because the concentration of solids, as
well as the aqueous solutions of products and excess reactants are neglible enough that the
mass and specific heat can be equal to the volume of water.
Question 3: Construct a plot of your temperature vs time data for Part 1, from Table 1
(using graphing format provided in Blackboard “How To” document and the supplementary
file (Experiment 1- Graph Extrapolation)). You can plot the graphs manually using the paper
template provided with Experiment 1 in Blackboard or you can use an appropriate graphing
program to complete them electronically. Use your prepared graph to show T
1
and T
2
using
the extrapolation process described in the instructions for the experiment. (See report note).
Include a copy of your completed graph with extrapolation.
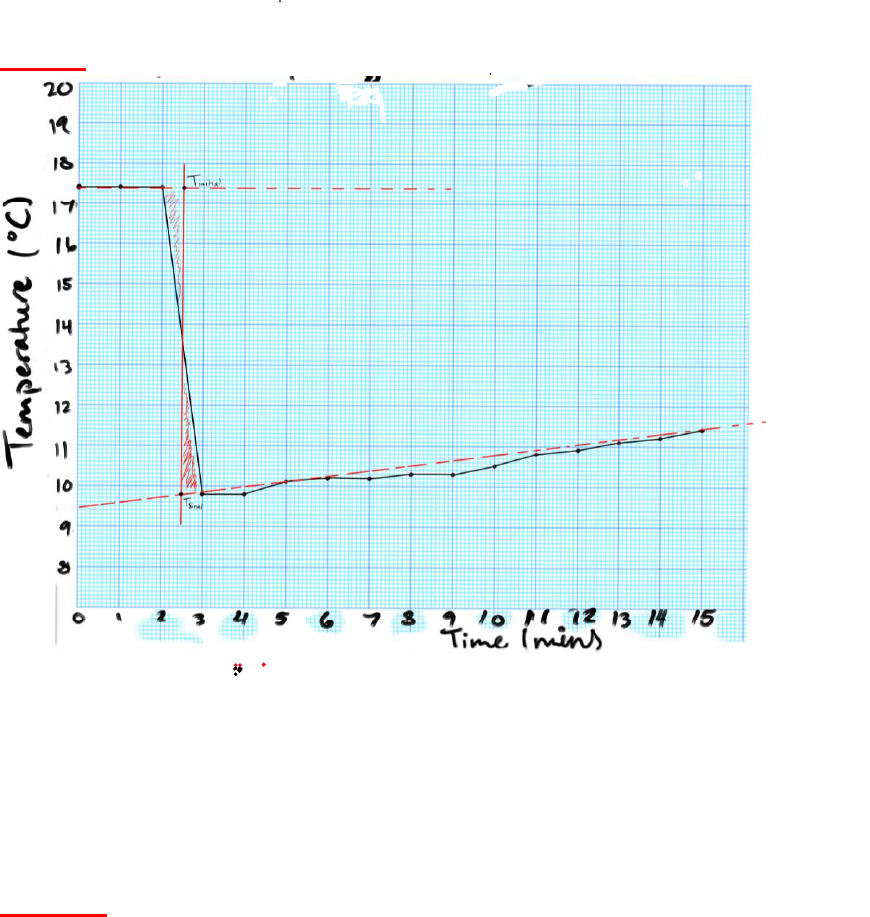
CHEM 1010 EXPERIMENT 1
4
GRAPH: Time vs. Temperature with Liquid Water and Ice
Using the known enthalpy of fusion (H
fus
) of ice, calculate the heat capacity of the
calorimeter with Eq. (5) from the laboratory instructions. (See report note – Part 1). Show
your complete calculation.
ANSWER: Therefore, the heat capacity of the calorimeter is 92.3 J/°C.

CHEM 1010 EXPERIMENT 1
5
Question 4: Construct a plot of your temperature vs time data for Part 2, from Table 2. Use
your prepared graph to show T
1
and T
2
using the extrapolation process described in the
instructions for the experiment. (See report note). Include a copy of your completed graph
with extrapolation.

CHEM 1010 EXPERIMENT 1
6
GRAPH:
Use Eq. (7) from the laboratory instructions to calculate the ∆H
rxn
of Mg reacting with the HCl
solution. (
Note: state any assumptions that have been made here
) (See report note – Part
2). Show your complete calculation.
ANSWER: Therefore, the enthalpy of the reaction is -518 kJ/mol.

CHEM 1010 EXPERIMENT 1
7
Using your data, calculate the heat of formation of Mg
2+
(aq)
,
i.e.
o
f
H
(Mg
2+
(aq)
), and the
reaction that is given below. (See report note)
Mg (s) + 2 H
+
(aq) → Mg
2+
(aq) + H
2
(g)
ANSWER: Therefore the heat of formation of Mg
2+
(aq)
is -467.0 kJ/mol.

CHEM 1010 EXPERIMENT 1
8
What indication is shown in the video that the reaction between Mg (s) and hydrochloric acid
is occurring?
ANSWER: The reaction between Mg (s) and hydrochloric acid is indicated by the gas being
released from the mixture
Question 5: Construct a plot of your temperature vs time data for Part 3, from Table 3. Use
your prepared graph to show T
1
and T
2
using the extrapolation process described in the
instructions for the experiment. (See report note). Include a copy of your completed graph
with extrapolation.
GRAPH:
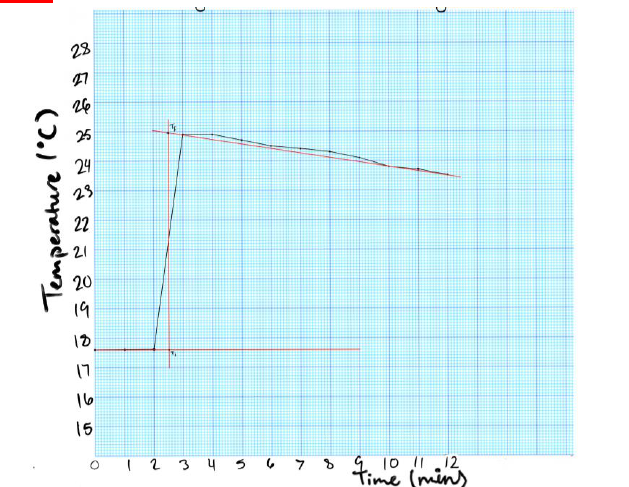
CHEM 1010 EXPERIMENT 1
9
Title: Time vs Temperature for Strong Acid (HCl) and Strong Base (NaOH)
Using your data, calculate the enthalpy of the overall reaction, ∆H
rxn
, for the neutralization of
HCl with NaOH, per 1 mole of HCl neutralized. (See report note – Part 3). Show your
complete calculation.
H
3
O
+
+ OH
-
→ 2 H
2
O

CHEM 1010 EXPERIMENT 1
10
ANSWER:
Given the definitions of strong acid and strong base, if you were to repeat this experiment
using HNO
3
as the acid, should you get the same value? Explain your reasoning.
ANSWER: If the experiment was repeated, using HNO
3
as the acid, it would not have a
lower enthalpy value, due to HCl being a stronger acid than nitric acid, and it would require
less energy to neutralize the solution.

CHEM 1010 EXPERIMENT 1
11
Question 6: Construct a plot of your temperature vs time data for Part 4, from Table 4. Use
your prepared graph to show T
1
and T
2
using the extrapolation process described in the
instructions for the experiment. (See report note). Include a copy of your completed graph
with extrapolation.
GRAPH:
Title: Time vs Temperature for Ammonium Nitrate in Water
Using your data, calculate the ∆H
rxn
for the aqueous dissociation of NH
4
NO
3
into NH
4
+
(aq)
and
NO
3
-
(aq)
, per mole of NH
4
NO
3
that dissociates. (See report note – Part 4). Show your
complete calculation.
ANSWER:
Therefore, the enthalpy of the reaction per mol of NH
4
NO
3
is -53.4 kJ/mol.

CHEM 1010 EXPERIMENT 1
12
Is this process exothermic or endothermic? Based on this answer, what molecules must be
supplying the necessary thermal energy in order for the dissociation reaction to occur?
ANSWER:
This process is endothermic. There is a positive enthalpy, so energy has to be put in to
break the hydrogen bonds from water (the bonds present initially are stronger than
bonds being formed). They supply the necessary thermal energy into the dissociation
reaction.

CHEM 1010 EXPERIMENT 1
13
References:
Textbook- section or page number
Blackboard- Chemistry 1010H
❑ Other (specify)
Works Cited
Chemistry: A molecular approach, 3
rd
Ed. [Online]; Tro, N. J.; Fridgen, T. D.; Shaw, L.; Pearson
Canada: Toronto, 2019; Chapter 6, pp 198–229. (accessed 01, February 2022).
Chemistry: A molecular approach, 3
rd
Ed. [Online]; Tro, N. J.; Fridgen, T. D.; Shaw, L.; Canada:
Toronto, 2019; Appendix II B. (accessed 01, February 2022).
CRC Density of Water Table [Online]; Blackboard, 2022. (accessed 01, February 2022).
Experiment 1 – Graph Extrapolation [Online]; Blackboard, 2022. (accessed 01, February 2022).
How to: Complete Report for First Year Chemistry [Online]; Blackboard, 2022. (accessed 01, February
2022).
Remote Experiment 1 – Calorimetry Data Set 5. Blackboard, 2022. (accessed 01, February 2022).
Chemistry: A molecular approach, 3
rd
Ed. [Online]; Tro, N. J.; Fridgen, T. D.; Shaw, L.; Pearson
Canada: Toronto, 2019; Chapter 6, pp 198–229. (accessed 01, February 2022).
Chemistry: A molecular approach, 3
rd
Ed. [Online]; Tro, N. J.; Fridgen, T. D.; Shaw, L.; Canada:
Toronto, 2019; Appendix II B. (accessed 01, February 2022).
