
AP Chemistry
Summer Reading and Assignment
PLEASE PICK UP PACKET IN FRONT OFFICE.
2023-24
Dr. Felicia Mitchell
Welcome to AP Chemistry! AP Chemistry builds on prior knowledge from Honors Chemistry.
You will need to spend the summer reviewing skills learned in Honors Chemistry. There will be
some things you will need to memorize to be successful in this course. Those items are
attached.
College Board organizes our material into 9 units. You will cover Unit 1 this summer at home.
When we meet in August, we will briefly discuss the unit, and you will be able to ask questions
in class. A test over Unit 1 will be given the second week of school. We will be moving quickly
through this unit to we make sure we have time to cover all of the material and leave time for
review before the AP exam next Spring.
You will watch the videos assigned and fill out the study guides as you watch. They will be
turned in for a daily grade the first day of class. Feel free to email me if you have any questions.
Expect to spend a total of about 2 hours on this part of the summer assignment. Each video is
about 15 minutes on average, and there are 9. So, you can easily do one a day and knock this
out in just a little over a week with a small time commitment each day. I recommend doing it in
July instead of June so it is fresh on your mind. The material in unit 1 is largely review, but a few
things are new material.
Links to the Unit 1 videos: Note:
You can search on You Tube: “Shanna Barkume AP Chemistry CED Unit 1” and view full playlist
and find the videos in order. They have a red/orange background.
1.1
https://www.youtube.com/watch?v=YBXHe3FKn3A&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAu
MAk4&index=1
1.2
https://www.youtube.com/watch?v=jjlkBCJ58lw&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuMAk
4&index=2

1.3
https://www.youtube.com/watch?v=1wIztEkLsY8&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuMA
k4&index=3
1.4
https://www.youtube.com/watch?v=hifgg0489nc&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuMA
k4&index=4
1.5 (2 videos)
https://www.youtube.com/watch?v=S6iNAvmnyG8&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAu
MAk4&index=5
https://www.youtube.com/watch?v=ntu3hwPo3ZE&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuM
Ak4&index=6
1.6
https://www.youtube.com/watch?v=acRPplg3l9k&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuMA
k4&index=7
1.7
https://www.youtube.com/watch?v=Q6IWuI4txdw&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuM
Ak4&index=8
1.8
https://www.youtube.com/watch?v=8al5h7ggpCQ&list=PLzFCBCoiEwAdzTtiGRh9_EU67okAuM
Ak4&index=9
Please work hard memorizing the monoatomic and polyatomic ions in this packet that need to
be committed to memory. Flash cards are a good suggestion. You will be allowed a periodic
table but it only has the symbols not element names. A great website to use for practice quizzes
is: https://www.sciencegeek.net/APchemistry/Quizzes/Ions/
You also need to memorize the six strong acids. A list of those is in this packet.

Recommended Optional AP Chemistry Review books:
Cracking the AP Chemistry Exam, (any year after 2014), by Paul Foglino, The Princeton Review
AP Chemistry Crash Course, 2nd Edition, by Adrian Dingle, Research & Education Association
Useful Websites:
https://www.khanacademy.org/science/ap-chemistry-
beta?msclkid=d0e4a375b2e511ec91a3d7dc303e3058
http://www.bozemanscience.com/ap-
chemistry/?msclkid=e5c5cdafb2e511ec85606598440ca907
https://www.sciencegeek.net/APchemistry/index.shtml?msclkid=fb152a71b2e511eca1c800d40
f9bcad5
I am excited to partner with you in this journey this year! I might be a pharmacist, but I started
as an education major. The Lord has always placed a love of teaching in my heart, and I am
grateful I get to share my passion for learning with you!

AP Chemistry Summer Assignment Packet
___________________________________________________________________
1. Memorize the following monoatomic and polyatomic ions. Be prepared for a quiz the
first few weeks of school.
Flash cards are a good suggestion. You will be allowed a periodic table (symbols only.) A great
website to use for practice quizzes is: https://www.sciencegeek.net/APchemistry/Quizzes/Ions/
acetate CH
3
COO
-
carbonate CO
3
2-
bicarbonate HCO
3
1-
nitrate NO
3
1-
sulfate SO
4
2-
hydroxide OH
1-
hydronium H
3
O
+
phosphate PO
4
3-
2. Memorize the following diatomic elements:
Hydrogen, oxygen, nitrogen, fluorine, chlorine, bromine, and iodine all form molecules of two
atoms of the same element.
H
2,
N
2,
O
2
, F
2
, Cl
2
, Br
2
, I
2
I’ll Have Neil Over For Clam Brains
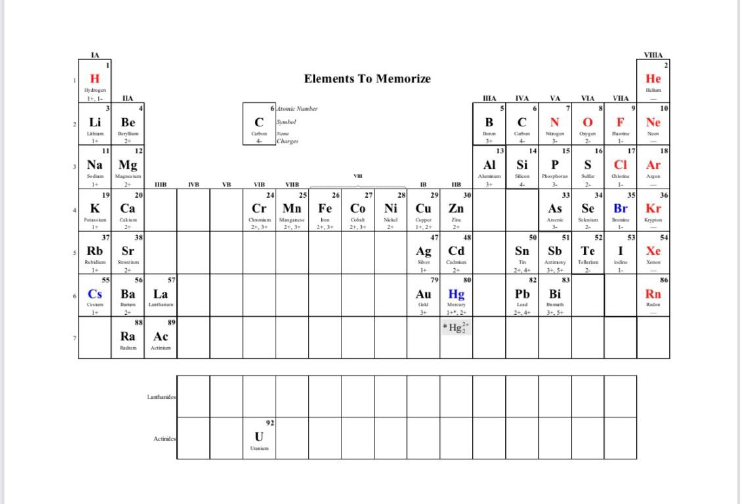
3. Try to memorize the following element names and symbols. This may take some time
over the first quarter.


