
Reference Manual for
Health Care Facilities with Limited Resources
Module 2. Hand Hygiene
Authors
Meredith A. Gerland, MPH, CIC
Bria S. Graham-Glover, MPH, CIC
Infection
Prevention
and Control.

The authors have made every effort to check the accuracy of all information, the dosages of any drugs, and
instructions for use of any devices or equipment. Because the science of infection prevention and control is rapidly
advancing and the knowledge base continues to expand, readers are advised to check current product information
provided by the manufacturer of:
• Each drug, to verify the recommended dose, method of administration, and precautions for use
• Each device, instrument, or piece of equipment to verify recommendations for use and/or operating
instructions
In addition, all forms, instructions, checklists, guidelines, and examples are intended as resources to be used and
adapted to meet national and local health care settings’ needs and requirements. Finally, neither the authors,
editors, nor the Jhpiego Corporation assume liability for any injury and/or damage to persons or property arising
from this publication.
Jhpiego is a nonprofit global leader in the creation and delivery of transformative health care solutions that save lives.
In partnership with national governments, health experts, and local communities, we build health providers’ skills,
and we develop systems that save lives now and guarantee healthier futures for women and their families. Our
aim is revolutionizing health care for the planet’s most disadvantaged people.
Jhpiego is a Johns Hopkins University affiliate.
Jhpiego Corporation
Brown’s Wharf
1615 Thames Street
Baltimore, MD 21231-3492, USA
www.jhpiego.org
© 2018 by Jhpiego Corporation. All rights reserved.
Editors: Melanie S. Curless, MPH, RN, CIC
Chandrakant S. Ruparelia, MD, MPH
Elizabeth Thompson, MHS
Polly A. Trexler, MS, CIC
Editorial assistance: Karen Kirk Design and layout: AJ Furay
Dana Lewison Young Kim
Joan Taylor Bekah Walsh
Module 2 Jhpiego technical reviewers: Chan Aung, Myanmar
Patricia Gomez, USA
Silvia Kelbert, USA

Infection Prevention and Control: Module 2 1
Module 2. Hand Hygiene
Chapter 1. Hand Hygiene .............................................................................................................................. 2
Key Topics ............................................................................................................................................... 2
Key Terms ............................................................................................................................................... 2
Background .................................................................................................................... ......................... 4
Hand Hygiene Opportunities .................................................................................................................. 4
Hand Hygiene Methods .......................................................................................................... ................ 5
Issues and Considerations Related to Hand Hygiene ........................................................................... 10
Monitoring Hand Hygiene .................................................................................................................... 12
Implementation of a Five-Step Hand Hygiene Improvement Strategy ................................................ 15
Summary............................................................................................................................................... 18
Appendix 1-A. Sample Hand Hygiene Observation Form: World Health Organization ....................... 19
Appendix 1-B. Sample Hand Hygiene Observation Form Modified for Room Entry and Exit .............. 22
Appendix 1-C. Implementation of a Multimodal Hand Hygiene Improvement Strategy ..................... 23
References ............................................................................................................................................ 27

Hand Hygiene
2 Infection Prevention and Control: Module 2, Chapter 1
Chapter 1. Hand Hygiene
Key Topics
Importance of hand hygiene
When to perform hand hygiene—the World Health Organization’s (WHO’s) “5 Moments for Hand
Hygiene”
Proper technique for washing hands with soap and water
Proper technique for use of alcohol-based handrub
Issues and considerations related to hand hygiene
Monitoring hand hygiene
WHO’s strategy for improving hand hygiene programs
Key Terms
Alcohol-based handrub (ABHR) is a fast-acting, antiseptic handrub that does not require water to
reduce resident flora, kills transient flora on the hands, and has the potential to protect the skin
(depending on the ingredients).
Antiseptic agents or antimicrobial soap (terms used interchangeably) are chemicals applied to the
skin or other living tissue to inhibit or kill microorganisms (both transient and resident). These
agents, which include alcohol (ethyl or isopropyl), dilute iodine solutions, iodophors, chlorhexidine,
and triclosan, are used to reduce the total bacterial count.
Antiseptic handwashing is washing hands with soap and water or with products containing an
antiseptic agent.
Clean water is natural or chemically treated or filtered water that is safe to drink and use for other
purposes (e.g., handwashing and general medical use) because it meets national public health
standards and the WHO guidelines for drinking-water quality.
Emollient is an organic agent (e.g., glycerol, propylene glycol, or sorbitol) that is added to ABHR to soften
the skin and help prevent skin damage (e.g., cracking, drying, irritation, and dermatitis) that is often
caused by frequent hand hygiene.
Hand disinfection is a term that WHO does not recommend using because disinfection normally refers
to the decontamination of non-living surfaces and objects.
Hand hygiene is the process of removing soil, debris, and microbes by cleansing hands using soap and
water, ABHR, antiseptic agents, or antimicrobial soap.
Handwashing is the process of mechanically removing soil, debris, and transient flora from hands using
soap and clean water.
Health care-associated infection (HAI) is an infection that occurs in a patient as a result of care at a
health care facility and was not present at the time of arrival at the facility. To be considered an HAI,
the infection must begin on or after the third day of admission to the health care facility (the day of
admission is Day 1) or on the day of or the day after discharge from the facility. The term “health
care-associated infection” replaces the formerly used “nosocomial” or “hospital” infection because
evidence has shown that these infections can affect patients in any setting where they receive
health care.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 3
Microorganisms are causative agents of infection, and include bacteria, viruses, fungi, and parasites.
Some bacteria can exist in a vegetative state (during which the organism is active and infective) and
as endospores (in which a tough, dormant, non-reproductive structure protects the cell).
Endospores are more difficult to kill due to their protective coating.
Persistent activity is prolonged or extended protective activity that prevents the growth or survival of
microorganisms after application of an antiseptic; it is also called “residual” activity.
Point of care is the place where three elements come together: the patient, the health care worker
(HCW), and the care or treatment involving contact with the patient or the surrounding environment. For
this chapter, the concept embraces the need to perform hand hygiene at recommended moments
exactly where care delivery takes place. This requires that a hand hygiene product (e.g., ABHR) be easily
accessible and as close as possible—within arm’s reach—to where patient care or treatment is provided.
Resident flora are microorganisms that live in the deeper layers of the skin and within hair follicles and
cannot be completely removed, even by vigorous washing and rinsing with plain soap and clean water. In
most cases, resident flora are not likely to be associated with infections; however, the hands or
fingernails of some HCWs can become colonized by microorganisms that do cause infection (e.g.,
Staphylococcus aureus, gram-negative bacilli, or yeast), which can be transmitted to patients.
Soap (term is used interchangeably with detergent) is a cleaning product (e.g., bar, liquid, leaflet, or
powder) that lowers surface tension of water, thereby helping to remove dirt and debris. Plain soaps do
not claim to be antimicrobial on their labels and require friction (i.e., scrubbing) to mechanically remove
microorganisms. Antiseptic (antimicrobial) soaps kill or inhibit growth of most microorganisms.
Standard Precautions are a set of infection control practices used for every patient encounter to
reduce the risk of transmission of bloodborne and other pathogens from both recognized and
unrecognized sources. They are the basic level of infection control practices to be used, at a
minimum, in preventing the spread of infectious agents to all individuals in the health care facility
(see Module 1, Chapter 2, Standard and Transmission-Based Precautions).
Surgical hand preparation refers to the protocol used preoperatively by surgical teams to eliminate
transient flora and reduce resident skin flora. The process involves an antiseptic handwash or
antiseptic handrub and rubbing/scrubbing for specific amounts of times using specific techniques
prior to donning gloves. Antiseptics used for surgical hand preparation often have persistent
antimicrobial activity (for details, see Module 7, Chapter 2, Use of Antiseptics in Health Care
Facilities):
Surgical handrub refers to surgical hand preparation with a waterless ABHR.
Surgical hand scrub refers to surgical hand preparation with antimicrobial soap and water.
Transient flora are microorganisms acquired through contact with individuals or contaminated
surfaces during the course of normal, daily activities. They live in the upper layers of the skin and are
more amenable to removal by hand hygiene. They are the microorganisms most likely to cause HAIs.
“The hands of healthcare workers are a major source of transmission of
nosocomial pathogens.”
–Bhalla et al. 2004

Hand Hygiene
4 Infection Prevention and Control: Module 2, Chapter 1
Background
Hand hygiene is the single most important measure to prevent transmission of infection and is the
cornerstone of infection prevention and control (IPC). The original study in this field was conducted at a
maternity hospital in Vienna, Austria, in 1847. This study demonstrated that the mortality rate among
mothers was significantly lower when the HCWs cleaned their hands with an antiseptic agent
(Semmelweiss 1861). Numerous other studies since then have demonstrated that HCWs’ hands become
contaminated during routine care of patients and can transmit infectious diseases from patient to patient
(AORN Recommended Practices Committee 2004; Duckro et al. 2005; Ojajarvi 1980; Pittet et al. 1999;
Riggs et al. 2007; Sanderson and Weissler 1992). Proper hand hygiene can prevent transmission of
microorganisms and decrease the frequency of HAIs. Despite evidence that hand hygiene prevents
transmission of infections, compliance with hand hygiene recommendations during patient care continues
to present ongoing challenges in all settings. Methods used to improve compliance with hand hygiene are
addressed later in this chapter.
The goal of hand hygiene is to remove soil, dirt, and debris and reduce both transient and resident flora.
Hand hygiene can be performed using ABHR or by washing hands with water and plain or antimicrobial
soap (bar or liquid) that contains an antiseptic agent such as chlorhexidine, iodophors, or triclosan. (WHO
2009a)
Traditionally, handwashing with soap and water has been the primary method of hand hygiene; however,
ABHR has been shown to be more effective for standard hand hygiene than plain or antimicrobial soaps.
(CDC 2002)
Recommendations for when and how to perform hand hygiene are described in this chapter. For
information and instructions about surgical hand scrub and surgical hand rub, see Module 7, Chapter 2,
Use of Antiseptics in Health Care Facilities.
“Failure to perform appropriate hand hygiene is considered to be the leading
cause of healthcare associated infections (HAIs) and the spread of multidrug
resistant microorganisms, and has been recognized as a significant contributor
to outbreaks.”
–Boyce et al. 2002
Hand Hygiene Opportunities
The World Health Organization has five recommended points in time when hand hygiene should occur in
order to prevent transmission of HAIs. These recommendations are called the “My 5 Moments for Hand
Hygiene” and focus on the following times:
1. Before making contact with a patient
2. Before performing a clean/aseptic task, including touching invasive devices
3. After performing a task involving the risk of exposure to a body fluid, including touching invasive
devices
4. After patient contact
5. After touching equipment in the patient’s surrounding areas (WHO 2006a)

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 5
The “5 Moments” are numbered according to health care workflow in an attempt to ease recall for HCWs
(see Figure 1-1).
Figure 1-1. WHO’s Five Recommended Moments for Hand Hygiene
Reprinted from: The “My 5 Moments for Hand Hygiene,” © World Health Organization (2009):
http://www.who.int/gpsc/5may/background/5moments/en/. Accessed June 28, 2016.
Hand Hygiene Methods
Handwashing with Soap and Water
The purpose of routine handwashing in health care is to remove dirt and organic material, as well
microbial contaminants, from the hands. Clean water must be used to prevent microorganisms in the
water from contaminating the hands. However, water alone is not effective at removing substances
containing fats and oils, which are often present on soiled hands. Proper handwashing also requires soap,
which is rubbed on all hand surfaces, followed by thorough rinsing and drying.
The cleansing activity of handwashing is achieved by both friction and the detergent properties of the
soap. Plain soap has minimal antimicrobial properties, but assists with the mechanical removal of debris
and loosely adherent microbes, while the mechanical action removes some bacteria from hands. Time is
also an important factor—handwashing for 30 seconds has been shown to remove 10 times the amount of
bacteria as handwashing for 15 seconds. The entire handwashing procedure, if completed properly, as
described step by step in Figure 1-2, should take 40–60 seconds. (CDC 2002; WHO 2009a)

Hand Hygiene
6 Infection Prevention and Control: Module 2, Chapter 1
Figure 1-2. The Steps for Routine Handwashing (How to Properly Wash Your Hands)
Reprinted from: “How to Handwash,” © World Health Organization (2009).
http://www.who.int/gpsc/5may/How_To_HandWash_Poster.pdf. Accessed May 6, 2016.
Handwashing with soap and water is recommended (rather than using ABHR) in the following situations:
If hands are visibly soiled or contaminated with blood or body fluids
After using the toilet
Before eating
To remove the buildup of emollients (e.g., glycerol) on hands after repeated use of ABHR
In outbreaks of C. difficile, but not in health care settings with only a few cases of C. difficile. (Cohen
et al. 2010; Siegel et al. 2007) C. difficile is a bacterial infection that causes severe diarrhea and is
common in some settings.
Avoiding contamination of hands during handwashing
Since microorganisms grow and multiply in moisture and in standing water, the following are
recommended to prevent contamination of hands during handwashing:
Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 7
Avoid bar soaps when possible because they can become contaminated, leading to colonization of
microorganisms on hands. There is some evidence, however, that the actual hazard of transmitting
microorganisms through handwashing with previously used bar soaps is negligible. If bar soap is
used, provide small bars and use soap racks that drain the water after use. (WHO 2009a)
Do not add liquid soap to a partially empty liquid soap dispenser. This is known as “topping off.” The
practice of topping off dispensers may lead to bacterial contamination of the soap. Using refill
packets avoids this problem but if they are not available, dispensers should be thoroughly cleaned
and dried before refilling. (WHO 2009a)
Filter and/or treat water if a health care facility’s water is suspected of being contaminated; this will
make the water microbiologically safer. (WHO 2009a) (See Module 5, Chapter 3, Managing Food and
Water Services for the Prevention of Health Care-Associated Infections, and Module 10, Chapter 6,
Preventing Health Care-Associated Infectious Diarrhea.)
Use running water for hand hygiene. In settings where no running water is available, water
“flowing” from a pre-filled container with a tap is preferable to still-standing water in a basin. Use a
container with a tap that can be turned off preferably with the back of the elbow (when hands are
lathered) and turned on again with the back of the elbow for rinsing. As a last resort, use a bucket
with a lid or a pitcher and a mug to draw water from the bucket, with the help of an assistant, if
available. (WHO 2009a)
Avoid dipping hands into basins of standing water. Even with the addition of an antiseptic agent
(e.g., Dettol or Savlon), microorganisms can survive and multiply in these solutions. (Rutala 1996)
If a drain is not available where hands are washed, collect water used from hand hygiene in a basin
and discard it in a drain or in a latrine.
Dry hands properly because wet hands can more readily acquire and spread microorganisms. Dry
hands thoroughly with a method that does not recontaminate the hands. Paper towels or single-use
clean cloths/towels are an option. Make sure that towels are not used multiple times or by multiple
individuals because shared towels quickly become contaminated. (WHO 2009a)
Alcohol-Based Handrub (ABHR)
The antimicrobial activity of alcohol results from its ability to denature proteins (i.e., the ability to dissolve
some microbe components) and kill microbes. Alcohol solutions containing 60–80% alcohol are most
effective, with higher concentrations being less effective. This paradox results from the fact that proteins
are not denatured easily in the absence of water; as a result, microorganisms are not killed as easily with
higher alcohol-based solutions (> 80% alcohol). (WHO 2009a)
The use of an ABHR is more effective in killing transient and resident flora than handwashing with
antimicrobial agents or plain soap and water. It also has persistent (long-lasting) activity. ABHR is quick and
convenient to use and can easily be made available at the point of care. ABHR usually contains a small
amount of an emollient (e.g., glycerol, propylene glycol, or sorbitol) that protects and softens skin. ABHR
should be used at any of the “5 Moments” described earlier in this chapter, unless hands are visibly soiled.
(CDC 2002; Girou et al. 2002; WHO 2009a)
To be effective, approximately 3–5 mL (i.e., 1 teaspoon) of ABHR should be used. The ideal volume of
ABHR to apply to the hands varies according to different formulations of the product and hand size (refer
to manufacturer’s instructions for use). ABHR should be used, following the steps shown in Figure 1-3, for
approximately 20–30 seconds or until the solution has fully dried. Since ABHR does not remove soil or
organic matter, if hands are visibly soiled or contaminated with blood or body fluids, handwash with soap

Hand Hygiene
8 Infection Prevention and Control: Module 2, Chapter 1
and water. To reduce the buildup of emollients on hands after repeated use of ABHR, washing hands with
soap and water after every 5–10 applications of ABHR is recommended.
In C. difficile outbreak settings, handwashing with soap and water is recommended over ABHR as it is more
effective than ABHR in removing endospores. If there are only a few cases of C. difficile, normal use of
ABHR is recommended (Cohen et al. 2010; Siegel et al. 2007; WHO 2009a). The need for using soap and
water over ABHR during outbreaks of norovirus is an unresolved issue. (Siegel et al. 2007; WHO 2009a)
Figure 1-3. WHO Recommendation on How to Perform Hand Hygiene with ABHR
Reprinted from: “How to handrub,” © World Health Organization (2009).
http://www.who.int/gpsc/5may/How_To_HandRub_Poster.pdf. Accessed May 6, 2016.
Producing alcohol-based handrub
An effective ABHR solution is inexpensive and simple to make, even in limited-resource settings. WHO
provides procedures for making ABHR in health care facility pharmacies (see Figure 1-4).

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 9
Figure 1-4. Alcohol-Based Handrub Formulation
Formulation 1: To produce final concentrations of ethanol 80% v/v, glycerol 1.45% v/v, hydrogen
peroxide (H
2
O
2
) 0.125% v/v:
Pour into a 1,000-mL graduated flask:
1. Ethanol 96% v/v, 833.0 mL
2. H
2
O
2
3%, 41.7 mL
3. Glycerol 98%, 14.5 mL
Top up the flask to 1,000 mL with distilled water or water that has been boiled and cooled; shake the
flask gently to mix the contents.
Formulation 2: To produce final concentrations of isopropyl alcohol 75% v/v, glycerol 1.45 v/v,
hydrogen peroxide 0.125% v/v:
Pour into a 1,000-mL graduated flask:
1. Isopropyl alcohol (with a purity of 99.8%), 751.5 mL
2. H
2
O
2
3%, 41.7 mL
3. Glycerol 98%, 14.5 mL
Top up the flask to 1,000 mL with distilled water or water that has been boiled and cooled; shake the
flask gently to mix the contents.
v/v=volume percent, meaning 80 parts absolute alcohol in volume and 20 parts water measured as
volume, not as weight
Adapted from:
WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge. Clean Care Is
Safer Care, page 49. © World Health Organization (2009).
Do not add ABHR to a partially empty dispenser. This practice of “topping off” dispensers may lead to
bacterial contamination. The use of refill packets avoids this problem but if they are not available, the
dispensers should first be thoroughly cleaned and dried before refilling. (WHO 2009a)
Antiseptic Soaps
Antiseptic soaps may be used in place of plain soap during the “My 5 Moments for Hand Hygiene”
described above but are not recommended for most settings. Handwashing with antiseptic soap is more
irritating to the skin and more expensive than using ABHR. Therefore, if available, ABHR should be used
under normal circumstances. (WHO 2009a)
Use of antiseptic soaps is recommended for surgical hand scrub and before entry into special areas of
health care facilities (e.g., neonatal intensive care units).
Surgical Hand Scrub
The purpose of the surgical hand scrub is to mechanically remove soil, dirt, debris, and transient flora
microorganisms and to reduce resident flora before and for the duration of the surgery. The goal is to
prevent wound contamination by microorganisms from the hands and arms of the surgical team members
(see Module 7, Chapter 2, Use of Antiseptics in Health Care Facilities, for more details).
Hand Hygiene
10 Infection Prevention and Control: Module 2, Chapter 1
Issues and Considerations Related to Hand Hygiene
Glove Use
While the effectiveness of gloves in preventing contamination of HCWs’ hands has been confirmed, gloves
do not provide complete protection against hand contamination. Contamination may occur as a result of
small, undetected holes in gloves, as well as during glove removal. Thus, wearing gloves does not replace
the need for proper hand hygiene. Hand hygiene should always be performed before putting on and after
removing gloves (see Module 3, Chapter 1, Personal Protective Equipment, for details of correct glove
use). (CDC 2002; WHO 2002)
Wearing the same pair of gloves and cleaning gloved hands between patients or between dirty and clean
body sites is not a safe hand hygiene practice (Siegel et al. 2007; WHO 2009a; WHO 2009c; WHO 2009d).
Not changing gloves between patients has been associated with transmission of microorganisms such as
methicillin-resistant S. aureus (MRSA) and gram-negative bacilli. Reprocessing gloves is not recommended.
Every effort must be made to reinforce the message that gloves do not replace the use of hand hygiene
and that when gloves are required, they should be used in addition to hand hygiene (see Module 3,
Chapter 1, Personal Protective Equipment, for more information on glove use).
Hand Lotions and Hand Creams
In an effort to minimize hand hygiene-related contact dermatitis (a skin rash caused by irritation from a
substance such as soap due to frequent hand hygiene), hand lotions, creams, barrier creams, and
moisturizing skin care products are recommended. Hand lotions and creams often contain humectants
(substances that help retain moisture) and various fats and oils. These humectants can increase hydration
and replace altered or depleted skin lipids that can serve as a barrier to microorganisms on normal skin.
Several studies have shown that regular use (i.e., at least twice per day) of such products can help prevent
and treat contact dermatitis. There is also biologic evidence that emollients (e.g., glycerol and sorbitol)
contained in ABHR, with or without antiseptics, may decrease cross-contamination because they reduce
shedding of bacteria from skin for up to 4 hours. These products are absorbed into the superficial layers of
the epidermis and are designed to form a protective layer that is not removed by standard handwashing.
(Boyce et al. 2002; McCormick et al. 2000; WHO 2009a)
Therefore, while use of hand lotions, creams, and moisturizers by HCWs should be encouraged there are
some considerations: First, to reduce the possibility of the products becoming contaminated, provide
small, individual-use containers or pump dispensers, which are completely emptied and cleaned before
being refilled. Refilling or topping off lotion containers may lead to contamination and proliferation of
bacteria within the lotion. Second, to avoid confusion, hand lotion dispensers should not be located near
dispensers of antiseptic solutions. Additionally, oil-based barrier products, such as those containing
petroleum jelly (e.g., Vaseline® or lanolin), should not be used because they damage latex rubber gloves.
Resistance to Topical Antiseptic Agents
With the increasing use of topical antiseptics, particularly in home settings, concern has been raised
regarding the development of resistance to these antiseptics by microorganisms. Although low-level
bacterial tolerance to commonly used antiseptic agents has been observed, studies have shown no clinical
evidence to date that supports the development of resistant microorganisms following use of any topical
antiseptic agents. (WHO 2009a)
Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 11
Lesions and Skin Breaks
Cuticles, hands, and forearms should be free of lesions (e.g., ulcers, abscesses, and tumors), dermatitis,
eczema, and skin breaks (e.g., cuts, abrasions, and cracking). Broken skin should be covered with
waterproof dressings. If covering is not possible, HCWs with active lesions should not perform clinical
duties until the lesions are healed. In particular, surgical HCWs with skin lesions should not operate until
the lesions are healed.
Religious and Cultural Considerations
It is clear that cultural and religious factors strongly influence attitudes toward handwashing. WHO’s
Guidelines on Hand Hygiene in Health Care provide information outlining these considerations. (WHO
2009a)
Fingernails
Research has shown that the area beneath the fingernails harbors the highest concentrations of bacteria
on the hands. This area most frequently harbors coagulase-negative staphylococci (a bacterium normally
found on the skin), gram-negative rods (bacteria known to cause infection), Corynebacteria (bacteria), and
yeasts. Fingernails longer than 0.2 cm (0.08 inches) have been shown to increase carriage rates of S.
aureus. Moreover, long nails, either natural or artificial, tend to puncture gloves more easily than short
nails. Therefore, nails should be kept moderately short—not extend more than 0.5 cm (0.2 inches) beyond
the fingertip. (CDC 2002; Fagernes and Lingaas 2011; McGinley et al. 1988; Olsen et al. 1993; WHO 2009a)
Artificial nails
Individuals with artificial nails have been shown to harbor more pathogenic organisms (i.e., disease-
causing microorganisms), especially gram-negative bacilli and yeast, on the nails and in the area beneath
the fingernails. Studies have demonstrated that the longer the artificial nail is, the more likely that a
pathogen can be isolated. Thus, artificial nails (e.g., nail wraps, nail tips, acrylic lengtheners) should not be
worn in clinical areas because they constitute an infection risk in high-risk areas. (Hedderwick et al. 2000;
Jumma 2005; Siegel et al. 2007)
Nail polish
Although there is no restriction on wearing nail polish, it is suggested that surgical HCWs and HCWs
working in specialty areas who want to use nail polish wear freshly applied, clear nail polish. There is
concern that individuals with fresh manicures may be hesitant to perform rigorous hand hygiene in an
effort to protect their nails, although no studies have demonstrated a relationship between freshly applied
nail polish and infection. But, compromises in hand hygiene technique may lead to transmission of
infection. Chipped nail polish supports the growth of larger numbers of organisms on fingernails compared
to freshly polished or natural nails. Also, dark-colored nail polish may prevent dirt and debris under
fingernails from being seen and removed. If nail polish is used, it should not be worn for more than 4 days.
At the end of 4 days, the nail polish should be removed and freshly reapplied, if necessary. (Baumgardner
et al. 1993; CDC 2002; Rothrock 2006)
Jewelry
Although current evidence demonstrates that wearing rings increases hand contamination, no studies
have related this to HCW-to-patient transmission of pathogens. Literature has shown that HCWs wearing
wristwatches had a higher total bacterial count on their hands compared to HCWs without wristwatches.
Surgical team members should not wear rings because it may be more difficult for them to put on surgical
gloves without tearing them. (Fagernes and Lingaas 2011; Siegel et al. 2007; Trick et al. 2003)

Hand Hygiene
12 Infection Prevention and Control: Module 2, Chapter 1
Monitoring Hand Hygiene
The WHO Guidelines on Hand Hygiene in Health Care encourage providers in all health care settings to
evaluate, improve, and monitor the reliability of hand hygiene practices with the aim of changing the
behavior of HCWs. Optimizing hand hygiene compliance at the 5 recommended moments for hand
hygiene increases patient safety. (WHO 2009a; WHO 2009e)
Hand hygiene compliance can be monitored both directly and indirectly (see Table 1-1) (WHO 2009a). Each
method of monitoring hand hygiene has its own advantages and disadvantages (see Table 1-2 for
advantages and disadvantages of each of the monitoring techniques). The direct observation of hand
hygiene compliance by a validated observer,
1
however, is considered the “gold standard” in hand hygiene
monitoring. It is often valuable to utilize more than one method of monitoring at the same time. (The Joint
Commission 2009; WHO 2009a)
Table 1-1. Hand Hygiene Observation Methods
Direct Methods of Hand Hygiene Observation Indirect Methods of Hand Hygiene Observation
Direct observation Monitoring consumption of products (soap or
ABHR)
Patient assessment Automated monitoring of use of sinks or ABHR
dispensers
In the implementation of a hand hygiene monitoring program, expectations for performing hand hygiene
should be clearly defined and made known within the health care facility. Policies detailing these
expectations should also be in place. Monitoring should occur on a regular, routine basis and a set
minimum number of observations should be collected in a given monitoring period.
1
Validated observers are observers with excellent skills in monitoring hand hygiene during health care practices. Validation
includes training according to the principles behind the “5 Moments,” training on facility policies related to hand hygiene
expectations, and being monitored and confirmed for correct techniques by senior observers. (WHO 2009a)

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 13
Table 1-2. Advantages and Disadvantages of Various Hand Hygiene Monitoring Approaches
Monitoring Approach Advantages Disadvantages
Direct observations by expert
observers
• Only way to reliably capture all
hand hygiene opportunities
• Details can be observed
• Unforeseen qualitative issues
can be detected while observing
hand hygiene
• Time-consuming
• Skilled and validated observers
required
• Prone to observation, observer, and
selection bias
Self-reports by HCWs
• Inexpensive • Overestimate of true compliance
• Not reliable
Direct observations by
patients
• Inexpensive • Potential negative impact on
patient-HCW relationship
• Reliability and validity required and
remain to be demonstrated
Consumption of hygiene
products (e.g., towels, soap,
and ABHR)
• Inexpensive
• Reflects overall hand hygiene
activity (selection biased)
• Validity may be improved by
using indirect denominators (e.g.,
patient-days or workload that is
converted into total hand
hygiene opportunities)
• Does not reliably measure the need
for hand hygiene (denominator)
• No information about the
appropriate timing of hand hygiene
actions
• Prolonged stocking of products at
ward level complicates and might
jeopardize the validity
• Validity threatened by increased
patient and visitor usage
• Not able to discriminate between
individual or professional group
usage
Reprinted from: WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge. Clean Care
Is Safer Care. Process and Outcome Measurement, page 162. © World Health Organization (2009).
Direct Monitoring
The goal of the direct hand hygiene observers is to observe HCWs during their usual patient care activities.
The observers should assess the HCWs’ compliance with indications for hand hygiene and with facility
policies on hand hygiene practices. It is preferable that observers have training and experience as patient
care professionals but this is not necessary.
Validity and reliability
2
are important aspects of direct hand hygiene monitoring. The validity of a new
observer should be confirmed by either joint observations with another confirmed observer or by being
tested through the WHO Training Film, which is available online. Results should be compared and any
discrepancies should be discussed. This process should be repeated until the HCW is fully competent.
(WHO 2009a)
2.
Validity—doing a procedure technically correctly following the “gold standard” for that procedure. Reliability—completing a
procedure technically correctly at all times following the “gold standard” for that procedure.
Hand Hygiene
14 Infection Prevention and Control: Module 2, Chapter 1
Hand hygiene observations should focus on the two essential parameters for determining hand hygiene
compliance:
1. The indication for hand hygiene
2. The observed hand hygiene action related to the indication
When the HCW is observed, the action is considered to have been either “performed” or “not performed.”
(WHO 2006b)
WHO recommends that the “5 Moments” be utilized as a framework for observing opportunities for hand
hygiene. It is possible, however, to simplify which moments are observed, based on the objectives of the
period of observation and/or the resources available. Observation can be limited to certain professional
role categories or disciplines or certain indications within the “5 Moments” (e.g., in some settings it may be
appropriate to observe the action of hand hygiene only before and after contact with the patient or the
patient environment). (WHO 2006b; WHO 2009a)
Observations should be collected in a standard way, such as on a form (see Appendix 1-A) with each hand
hygiene observation session on a separate form. A standard form should have three main sections:
1. A header containing information about the health care facility and the location within the facility
where the session was completed
2. A second header containing information on the session observed
3. Columns below the headers representing the sequence of actions for different HCWs observed
during the same session, with each column representing one HCW (See Appendix 1-A for the WHO
Observation Form – Short Description of Items on the Form.) (WHO 2009a; WHO 2009e)
Content can be adapted to suit the needs of the facility. Appendix 1-B is a sample observation form for
hand hygiene data collection. This form reflects a modified approach that looks at hand hygiene
compliance at room entry and room exit only (useful for areas with single-patient rooms).
Hand hygiene compliance (%) is the simplest way to analyze the hand hygiene data collected. Hand
hygiene compliance is the ratio of the number of actions to the number of opportunities:
Compliance (%) = (# of Hand Hygiene Actions/Total # of Opportunities) x 100
Compliance data can be summarized based on total compliance by HCW, by role or discipline (e.g.,
doctors, nurses), or by location (e.g., ward A, ward B), depending on the objectives of the monitoring
program. It is important to provide feedback and disseminate compliance data to the HCWs and leaders
after the observation session/assessments are completed. Minimizing the delay between observation and
reporting of results may help increase the effects of the monitoring. (WHO 2009a)
There are some limitations with direct monitoring of hand hygiene. For example, HCWs may improve or
modify their behavior in response to being observed or studied, resulting in an overestimate of
compliance. Thus, it is important to be aware of this effect when evaluating compliance rates.
Indirect Monitoring
Indirect hand hygiene monitoring, such as monitoring the consumption of hand hygiene products (e.g.,
soap, ABHR, paper towels) to estimate the number of hand hygiene actions, is a less expensive monitoring
approach and can be useful in settings where resources for direct monitoring are limited. However, this

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 15
methodology requires validation to be most effective. One of the major limitations to this type of indirect
monitoring is that it is impossible to determine if the hand hygiene actions were performed at the proper
moment. (WHO 2009a)
Implementation of a Multimodal Hand Hygiene Improvement Strategy
The WHO Multimodal Hand Hygiene Improvement Strategy identifies key components to address during
the implementation of a hand hygiene improvement strategy. (WHO 2009a; WHO 2009e) (See Appendix 1-
C.) The component are:
• System change to ensure that infrastructure is in place, including availability of ABHR and access to a
safe and continuous supply of water, soap, and towels—to allow HCWs to practice hand hygiene
• Training and education of HCWs
• Monitoring of hand hygiene practices and provision of feedback
• Reminders in the workplace
• Creation of a safety culture
In order to implement these components, the guidelines detail five sequential steps, listed below, with
each step building on the activities and actions in the previous steps (see Figure 1-5). Rather than a linear
process, the five steps should be considered a cyclical process, with each cycle being repeated, refined,
and enhanced at least every 5 years. It is imperative to evaluate success factors and areas of weakness
within the program in order to achieve long-term sustainability and process improvement. (WHO 2009a)
Figure 1-5. Five Steps of the Hand Hygiene Improvement Strategy
Adapted from: WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge: Clean Care Is
Safer Care. The WHO Multimodal Hand Hygiene Improvement Strategy, page 99. © World Health Organization
(2009).
Although complex, the hand hygiene improvement strategy lays the groundwork for the implementation
of a sustainable hand hygiene monitoring program. It is aimed at improving hand hygiene compliance and
Hand Hygiene
16 Infection Prevention and Control: Module 2, Chapter 1
increasing patient safety in the health care facility. The basic elements of each step are listed below. (WHO
2009a; WHO 2009e)
Step 1: Facility Preparedness
Assess and ensure the preparedness of the health care facility. Consider the following:
Identify a person or team to coordinate the program.
Identify HCWs and facility leadership who will play a major role in program implementation.
Obtain raw materials to produce ABHR at the health care facility’s pharmacy (if necessary).
Train observers on how to monitor hand hygiene practices.
Train identified persons on how to calculate hand hygiene compliance.
Step 2: Baseline Evaluation
Include a baseline evaluation of hand hygiene practices, facility infrastructure, HCW knowledge, and
current beliefs about hand hygiene. Consider the following:
Survey HCWs on their perceptions of hand hygiene (e.g., do they think hand hygiene is important,
and/or effective, and/or necessary?).
Survey HCWs on their knowledge of hand hygiene (e.g., do they know how and when to perform
proper hand hygiene?).
Look for details in the health care facility’s structure that may help explain current hand hygiene
compliance (e.g., is there easy access to running water, sinks, and/or ABHR?).
Monitor use of soap and ABHR, if applicable.
Collect baseline data on hand hygiene compliance.
Make sure that ABHR and dispensers are available in time for the start of Step 3.
Compile data on hand hygiene practices.
Step 3: Implementation
Implement the planned program. Consider the following:
Share baseline data with HCWs.
Distribute educational materials, hand hygiene guidelines, and/or policies to HCWs.
Distribute ABHR to HCWs.
Measure how much ABHR is used each month.
Hold education and training sessions.
Survey HCWs on their opinion of the ABHR (e.g., do they find it acceptable?).
Continue to monitor hand hygiene compliance observations, if possible.
Meet monthly with key HCWs involved with the hand hygiene program.
Step 4: Follow-Up Evaluation
Evaluate the short-term impact of the implemented hand hygiene program. Considered the following:

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 17
Survey HCWs and health care facility leadership on their perceptions of hand hygiene (e.g., do they
think hand hygiene is important and/or effective and/or necessary?).
Survey HCWs on their knowledge of hand hygiene (e.g., do they know how and when to perform
proper hand hygiene?).
Inspect the health care facility structure to determine if there are still any barriers to hand hygiene
compliance related to structural issues.
Collect data on soap and ABHR use.
Collect data on hand hygiene compliance.
Complete data entry.
Step 5: Development of an Ongoing Action Plan and Review Cycle
Develop an ongoing action plan and review cycle. Consider the following:
Review collected data and results carefully.
Prepare a report of the findings of the entire program.
Share information about the findings of the program with leadership and HCWs.
Create a 5-year plan of action to continue to improve and promote hand hygiene compliance.
Modifying a Hand Hygiene Program
In situations where the complete implementation of the WHO hand hygiene improvement strategy is not
possible, due to either limited resources or time, a hand hygiene improvement team should focus on the
minimum criteria listed below (see Table 1-3). These criteria ensure achievement of each component of
the multimodal strategy and include the most pertinent steps of the program. (WHO 2009a)
Table 1-3. Minimum Criteria for Implementation
Multimodal Component Minimum Criteria for Implementation
1a. System change: ABHR Bottles of ABHR are positioned at the point of care in each
ward or given to HCWs.
1b. System change: Access to safe, continuous
water supply and towels
There is one sink for at least every 10 beds; soap; running
water; and clean, dry towels available at every sink.
2. Training and education A program to update training over the short, medium, and
long term is established.
3. Observation and feedback Two periods of observational monitoring are undertaken, the
baseline evaluation and the follow-up evaluation.
4. Reminders in the workplace “How to” and “5 Moments” posters are displayed in all
wards (e.g., patient rooms, health facility staff areas,
outpatient areas, ambulatory departments).
5. Institutional safety climate The chief executive, chief medical officer/medical
superintendent, and chief nurse all make a visible
commitment to support hand hygiene improvement during

Hand Hygiene
18 Infection Prevention and Control: Module 2, Chapter 1
program implementation (e.g., verbal announcements
and/or formal letters to health facility staff).
Reprinted from: Guide to Implementation: A Guide to the Implementation of the WHO Multimodal Hand Hygiene
Improvement Strategy, page 39. © World Health Organization (2009).
Summary
Hand hygiene is the single most important measure to prevent transmission of infection and is the
cornerstone of IPC. The goal of hand hygiene in health care is to prevent transmission of infections through
removing bacteria from hands at strategic “moments” during the care of patients. Hand hygiene can be
performed using ABHR or by washing hands with water and soap. ABHR has been shown to be more
effective for standard hand hygiene than plain or antimicrobial soaps and more easily available at the point
of care. Despite evidence proving that hand hygiene prevents transmission of infections, compliance with
hand hygiene recommendations during patient care continues to be challenging in all settings and requires
constant and ongoing efforts from IPC staff. The WHO Multimodal Hand Hygiene Improvement Strategy
provides a guide for implementation of a sustainable hand hygiene program at health care facilities.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 19
Appendix 1-A. Sample Hand Hygiene Observation Form:
World Health Organization
Source: WHO 2009e.

Hand Hygiene
20 Infection Prevention and Control: Module 2, Chapter 1
Source: WHO 2009e.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 21
Source: WHO 2009e.
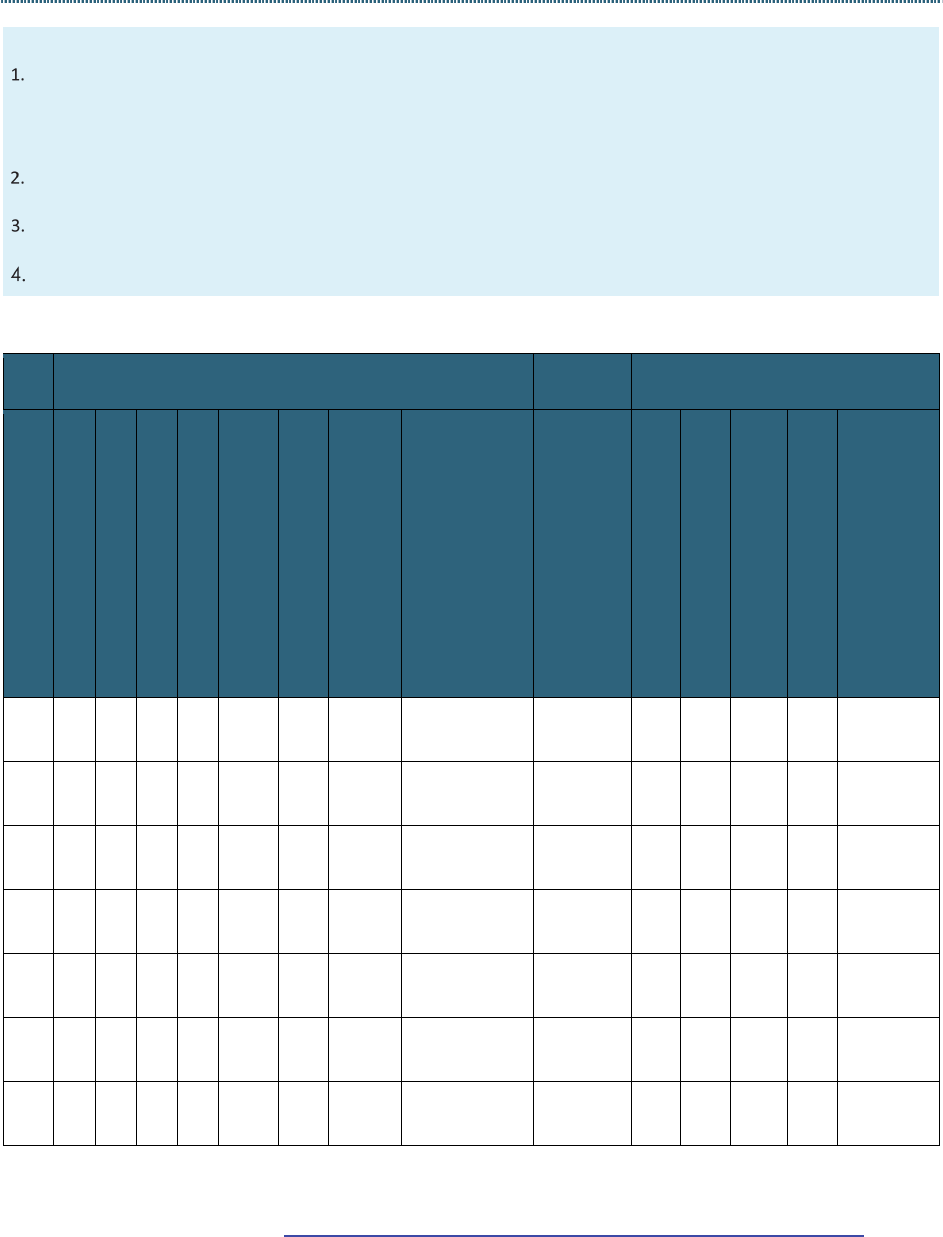
Hand Hygiene
22 Infection Prevention and Control: Module 2, Chapter 1
Appendix 1-B. Sample Hand Hygiene Observation Form
Modified for Room Entry and Exit
FOUR rules for conducting Hand Hygiene Observations
Observe for hand hygiene upon ENTRY and EXIT from Patient Environment
Patient Environment definition:
Private or semi-private room: Crossing room door
Between patients and multi-patient rooms setting: Crossing the “curtain line”
A provider may use the alcohol-based handrub (ABHR) dispenser just outside the room door, inside the room, at the
sink, or the health care worker’s personal ABHR bottle.
DO NOT GUESS. If your view is blocked and you cannot confirm if provider performed hand hygiene, simply check
“Unsure” box.
Do not exceed 3 observations per provider in one session.
UNIT:______________________ DATE:____/_____/____ DAY OF WEEK:_______________
TIME: ________ TO __________ OBSERVER NAME: __________________________________________
Role of Observed Person
Hand
Hygiene
Observed Behavior
Obs #
Nurse*
Midwife
Physicians (all doctors)
CO/PA/Dentist**
Pharmacist/Laboratory Technician
Support Staff
Other Providers (nursing, medical
and other students, and residents)
Other
1=Unknown
2=Clinical
procedure
3=Transport
4=Nursing
care
5=Blood
sample
collection
6= Nutrition
7= Admin
Circle
ONE
Not observed
Hand cleaning with ABHR
Hand wash with soap and water
No hand hygiene
Area location
ENTRY
EXIT
ENTRY
EXIT
ENTRY
EXIT
ENTRY
EXIT
ENTRY
EXIT
ENTRY
EXIT
ENTRY
EXIT
* All types of nursing staff including diploma, degree, post-graduate, supervisor, and assistant.
** CO=Clinical Officer, PA=Physician Assistant.
Adapted from: Johns Hopkins Medicine. Hospital Epidemiology and Infection Control. JHH Hand Hygiene
Compliance Data Collection Form. http://www.hopkinsmedicine.org/heic/docs/HH_observation_form.pdf.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 23
Appendix 1-C. Implementation of a Multimodal Hand
Hygiene Improvement Strategy
As discussed in this Hand Hygiene chapter, the WHO Multimodal Hand Hygiene Improvement Strategy
identifies five key steps to implement a hand hygiene improvement strategy (see Steps 1–5 below). The
implementation strategy was developed based on a literature review of the implementation science,
behavioral change, spread methodology, diffusion of innovation, and impact evaluation (WHO 2009a). For
detailed information on assessing the economic impact of hand hygiene promotion, refer to WHO
Guidelines on Hand Hygiene in Health Care, page 168.
It is important to note that although each step within the process builds upon activities occurring in
previous steps, it should be considered a cyclical process rather than a linear one. Each step of the cycle
should be repeated, refined, and enhanced at least every 5 years in order to maximize the impact of the
hand hygiene program. (WHO 2009a; WHO 2009f)
Step 1: Facility Preparedness
Suggested duration: 3 months
Step 1 in the hand hygiene improvement strategy is to evaluate and prepare the facility for the program.
To have a successful hand hygiene program, careful planning is required from the start of the program.
During Step 1, it is imperative to map out a clear strategy for the entire program.
Step 1: Key Activities in Facility Preparedness
Key Activities
Identify coordinator.
Identify key individuals/groups.
Undertake a situation analysis of hand hygiene practices at the facility.
3
Complete ABHR production, planning, and costing tool.
Train observers/trainers.
Procure raw materials for ABHR (if necessary).
Collect data on costs/benefits of hand hygiene improvement program: costs of program versus reductions in
costs of managing hospital acquired infections.
Undertake training on data entry and analysis.
Steps 1–5 Reproduced from: WHO Guidelines on Hand Hygiene in Health Care, page 119. © World Health
Organization (2009): http://apps.who.int/iris/bitstream/10665/44102/1/9789241597906_eng.pdf. Accessed May
6, 2016.
3
See as an example: WHO. 2010. WHO Hand Hygiene Self-Assessment Framework 2010.
http://www.who.int/gpsc/country_work/hhsa_framework_October_2010.pdf?ua=1.

Hand Hygiene
24 Infection Prevention and Control: Module 2, Chapter 1
Step 2: Baseline Evaluation
Suggested duration: 2–3 months
Step 2 includes the baseline evaluation of hand hygiene practices, perceptions, knowledge, and available
infrastructure within the health care facility.
Hand hygiene is the most effective way of preventing the transmission of infections and it is imperative to
collect data on HCWs’ perception on the importance of hand hygiene. These perceptions, as well as other
factors influencing compliance, will provide valuable information for strategy development. Changing
perceptions can be the means by which improvements in hand hygiene practices are achieved. Similarly,
assessing the infrastructure of the health care facility may help explain current hand hygiene practices and
will guide improvement efforts. Lack of access to sinks, running water, and ABHR may all contribute to low
hand hygiene compliance and should be addressed during the implementation planning step.
Step 2: Key Activities in Baseline Evaluation
Key Activities
Undertake baseline assessments:
• Senior manager perception survey
• HCW perceptions survey
• Ward structure survey
• HCW knowledge survey
Begin local production or market procurement of ABHR.
Conduct hand hygiene observations.
Monitor use of soap and ABHR.
Perform data entry and analysis.
Step 3: Implementation
Suggested duration: 3–4 months
Step 3 is implementation of the planned program. Availability of ABHR at the point of care and education
and training for HCWs are crucial to the success of this step. Health care facilities may choose to hold a
high-profile launch event to coincide with the start of the program’s implementation. Publicizing
leadership endorsement and support also helps foster a successful implementation stage (WHO 2009a).
During implementation, it is also important to evaluate HCWs’ tolerance and acceptance of ABHR. Monthly
collection of hand hygiene observations should continue during implementation, if possible. If time and
resources are limited, observations should occur only during Step 2 and Step 4.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 25
Step 3: Key Activities in Implementation
Key Activities
Launch the strategy.
Provide feedback on baseline data.
Distribute posters.
Distribute ABHR.
Distribute other WHO materials from the Pilot Implementation Pack.
Educate HCWs.
Undertake practical training of HCWs.
Undertake ABHR tolerance tests.
Complete monthly monitoring of usage of products.
Step 4: Follow-Up Evaluation
Suggested duration: 2–3 months
Step 4 is the evaluation of the short-term impact of the hand hygiene improvement strategy. By
performing a follow-up evaluation, facilities will gain information they can use to make future decisions
and take actions related to the hand hygiene program. Compliance with hand hygiene practices among
HCWs is the main indicator that should be evaluated. It is important to note that hand hygiene
improvement activities should continue in the health care facility according to the local action plan, even
during this evaluation step.
WHO has identified the following as key success indicators in the evaluation of the short-term impact of a
hand hygiene program:
Increase in hand hygiene compliance
Improvement in infection control/hand hygiene structures
Increase in usage of hand hygiene products
Improved perception of hand hygiene
Improved knowledge of hand hygiene
The data collected during this evaluation will help shape future actions and the steps the health care
facility may take to maintain high hand hygiene compliance rates over time.

Hand Hygiene
26 Infection Prevention and Control: Module 2, Chapter 1
Step 4: Key Activities in Follow-Up Evaluation
Key Activities
Undertake follow-up assessments:
• HCW knowledge survey
• Senior executive manager perception survey
• HCW perception and campaign evaluation survey
• Facility situation analysis
Conduct data entry and analysis.
Conduct hand hygiene observations.
Continue monthly monitoring of use of products.
Step 5: Developing Ongoing Action Plan and Review Cycle
Suggested duration: 2–3 months
Step 5 is to develop an ongoing action plan and review cycle. The goal of the hand hygiene program is to
create an environment in which performing appropriate hand hygiene is central to the facility’s culture.
Reviewing the results of the data and creating a final report detailing the results of the improvement
program will help condense the findings and will aid in creating a future action plan. Enthusiasm and
motivation for the program must remain high in order to have long-term impacts.
Step 5: Key Activities in Developing an Ongoing Action Plan and Review Cycle
Key Activities
Study all results carefully.
Provide follow-up data.
Develop a 5-year action plan.
Consider scale-up of the strategy.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 27
References
Association for Professionals in Infection Control and Epidemiology (APIC). 2014. APIC Text of Infection
Control and Epidemiology, 4th ed. Washington, DC: APIC.
Association of periOperative Registered Nurses (AORN) Recommended Practices Committee. 2004.
Recommended practices for surgical hand antisepsis/hand scrub. AORN J. 79(2):416, 418, 421–424, 426,
429–431.
Baumgardner CA, Sallese Marago C, Walz JM, Larson E. 1993. Effects of nail polish on microbial growth of
fingernails: dispelling sacred cows. AORN J. 58(1):84–88.
Bhalla A, Pultz NJ, Gries DM, HICPAC/SHSA/APIC/IDSA Hand Hygiene Task Force. 2004. Acquisition of
nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients.
Infect Control Hosp Epidemiol. 25(2):164–167.
Boyce JM, Pittet D, the Healthcare Infection Control Practices Advisory Committee. 2002. Guideline for
hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices
Advisory Committee and the HICPAC/SHSA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp
Epidemiol. 23(Suppl 12):S3–S40.
Centers for Diseases Control and Prevention (CDC). 2002. Guideline for hand hygiene in health-care settings:
recommendations of the Healthcare Infection Control Practices Advisory Committee and the
HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR. 51(RR-16).
http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf.
CDC. 2016. CDC/NHSN [National Healthcare Safety Network] Surveillance Definitions for Specific Types of
Infections. http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf.
Cohen SH, Gerding DN, Johnson S, et al. 2010. Clinical practice guidelines for Clostridium difficile infection
in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious
Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 31(5):431–455.
Duckro AN, Blom DW, Lyle EA, Weinstein RA, Hayden MK. 2005. Transfer of vancomycin-resistant
Enterococci via health care worker hands. Arch Intern Med. 165(3):302–307.
Fagernes M, Lingaas E. 2011. Factors interfering with the microflora on hands: a regression analysis of
samples from 465 healthcare workers. J Adv Nurs. 67(2):297–307.
Girou E, Loyeau S, Legrand P, Oppein R, Brun-Buisson C. 2002. Efficacy of handrubbing with alcohol based
solution versus standard handwashing with antiseptic soap: randomized clinical trial. BMJ. 325(7360):362–
365.
Hedderwick SA, McNeil SA, Lyons MJ, Kauffman CA. 2000. Pathogenic organisms associated with artificial
fingernails worn by healthcare workers. Infect Control Hosp Epidemiol. 21(8):505–509.
Johns Hopkins Medicine Department of Hospital Epidemiology and Infection Control. n.d. Hand Hygiene
Compliance Data Collection Form.
http://www.hopkinsmedicine.org/heic/docs/HH_observation_form.pdf.
The Joint Commission. 2009. Measuring Hand Hygiene Adherence: Overcoming the Challenges. Oakbrook
Terrace, IL: The Joint Commission.
Jumma PA. 2005. Hand hygiene: simple and complex. Int J Infect Dis. 9(1):3–14.
Larson E. 1988. A causal link between handwashing and risk of infection? Examination of the evidence.
Infect Control Hosp Epidemiol. 9(1):28–36.
Larson E. 1999. Skin hygiene and infection prevention: more of the same or different approaches? Clin
Infect Dis. 29(5):1287–1294.

Hand Hygiene
28 Infection Prevention and Control: Module 2, Chapter 1
McCormick RD, Buchman TL, Maki DG. 2000. Double-blind, randomized trial of scheduled use of a novel
barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect
Control. 28(4):302–310.
McGinley KJ, Larson EL, Leydon JJ. 1988. Composition and density of microflora in the subungual space of
the hand. J Clin Microbiol. 26(5):950–953.
Ojajarvi J. 1980. Effectiveness of handwashing and disinfection methods in removing transient bacteria
after patient nursing. J Hyg (Lond). 85(2):193–203.
Olsen RJ, Lynch P, Coyle MB, et al. 1993. Examination gloves as barriers to hand contamination in clinical
practice. JAMA. 270(3):350–353.
Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. 1999. Bacterial contamination of the hands of
hospital staff during routine patient care. Arch Intern Med. 159(8):821–826.
Riggs MM, Sethi AK, Zabarsky TF, et al. 2007. Asymptomatic carriers are a potential source for transmission
of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin
Infect Dis. 45(8):992–998.
Rothrock J. 2006. What Are the Current Guidelines about Wearing Artificial Nails and Nail Polish in the
Healthcare Setting? http://www.medscape.com/viewarticle/547793.
Rutala WA. 1996. Association for Professionals in Infection Control and Hospital Epidemiology (APIC)
guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. APIC.
Amer J Infect Control. 24(4):313–342.
Sanderson PJ, Weissler S. 1992. Recovery of coliforms from the hands of nurses and patients: activities
leading to contamination. J Hosp Inf. 21(2):85–93.
Semmelweiss IP. 1861. Die Aetologie, der Begriff und die Prophylaxis Des Kindbettfiebers. Pest, Hungary:
CA Hattleberi’s Verlags-Expeditions.
Siegel JD, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory
Committee (HICPAC). 2007. Guideline for Isolation Precautions: Preventing Transmission of Infectious
Agents in Healthcare Settings. Atlanta, GA: CDC/HICPAC.
http://www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html.
Trick WE, Vernon MO, Hayes RA, et al. 2003. Impact of ring wearing on hand contamination and
comparison of hand hygiene agents in a hospital. Clin Infect Dis. 36(11):1383–1390.
World Health Organization (WHO). 2002. Prevention of Hospital-Acquired Infections: A Practical Guide, 2nd
ed. http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_EPH_2002_12/en/.
WHO. 2006a. Hand Hygiene: When and How. http://www.who.int/gpsc/tools/Pocket-Leaflet.pdf?ua=1.
WHO. 2006b. Hand Hygiene Technical Reference Manual: To Be Used by Health-care Workers, Trainers and
Observers of Hand Hygiene Practices. Geneva, Switzerland: WHO.
http://whqlibdoc.who.int/publications/2009/9789241598606_eng.pdf.
WHO. 2009a. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge. Clean
Care Is Safer Care. Geneva, Switzerland: WHO.
http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf.
WHO. 2009b. Definition of terms. In: WHO Guidelines on Hand Hygiene in Health Care: First Global Patient
Safety Challenge. Clean Care Is Safer Care. Geneva, Switzerland: WHO.
http://www.ncbi.nlm.nih.gov/books/NBK144046/.

Hand Hygiene
Infection Prevention and Control: Module 2, Chapter 1 29
WHO. 2009c. How to Handwash. http://www.who.int/gpsc/5may/
How_To_HandWash_Poster.pdf.
WHO. 2009d. Glove Use Information Leaflet. http://www.who.int/
gpsc/5may/Glove_Use_Information_Leaflet.pdf.
WHO. 2009e. Patient Safety. Saves Lives; Clean Your Hands Observation Form.
http://www.who.int/gpsc/5may/Observation_Form.doc.
WHO. 2009f. Guide to Implementation: A Guide to the Implementation of the WHO Multimodal Hand
Hygiene Improvement Strategy. Geneva, Switzerland: WHO.
http://www.who.int/gpsc/5may/Guide_to_Implementation.pdf.
WHO. 2010a. Hand Hygiene Self-Assessment Framework 2010: Introduction and User Instructions.
http://www.who.int/gpsc/country_work/hhsa_framework_October_2010.pdf?ua=1.
WHO. 2010b. Water for Health: WHO Guidelines for Drinking-Water Quality.
http://www.who.int/water_sanitation_health/WHS_WWD2010_guidelines_2010_6_en.pdf? ua=1.
WHO. 2011. Report on the Burden of Endemic Health Care-Associated Infection Worldwide: Clean Care Is
Safer Care. A Systematic Review of the Literature. Geneva, Switzerland: WHO.
http://apps.who.int/iris/bitstream/10665/80135/1/9789241501507_eng.pdf.
WHO. n.d. Training Film: A Tool to Help Convey the Concept of the “5 Moments for Hand Hygiene” to
Health-Care Workers. http://www.who.int/gpsc/media/training_film/en/.
Hand Hygiene
30 Infection Prevention and Control: Module 2, Chapter 1
