
1
Purpose
This AUA Guidelines focuses on the evaluation and management of clinically
localized sporadic renal masses suspicious for renal cell carcinoma (RCC) in adults,
including solid enhancing renal tumors and Bosniak 3 and 4 complex cystic renal
masses. Some patients with clinically localized renal masses may present with
findings suggesting aggressive tumor biology or may be upstaged on exploration
or final pathology. Management considerations pertinent to the urologist in such
patients will also be discussed. The follow-up of renal cancer patients after
intervention is also addressed, including recommendations for periodic clinical
follow-up and abdominal and chest imaging. Practice patterns regarding such
tumors vary considerably, and the literature regarding evaluation, management,
and surveillance has been rapidly evolving. Notable examples include
controversies about the role of renal mass biopsy (RMB) and concerns regarding
overutilization of radical nephrectomy (RN). Please also refer to the associated
Renal Mass and Localized Renal Cancer Treatment and Follow-up after
Intervention algorithms.
Methodology
The systematic review utilized in the creation of this guideline was completed in
part through the Agency for Healthcare Research and Quality (AHRQ) and through
additional supplementation that further addressed additional key questions and
more recently published literature. A research librarian experienced in conducting
literature searches for comparative effectiveness reviews searched in MEDLINE®,
Embase®, the Cochrane Library, the Database of Abstracts of Reviews of Effects,
the Health Technology Assessment Database, and the UK National Health Service
Economic Evaluation database to capture both published and gray literature
published from January 1, 1997 through May 1, 2015. A supplemental search was
conducted adding additional literature published through August 2015, and a final
update search was conducted through July 2016.
A systematic review was conducted in 2013 to identify published articles relevant
to key questions specified by the Panel related to kidney neoplasms and their
follow-up (imaging, renal function, markers, biopsy, and prognosis). This search
covered English-language articles published between January 1999 and 2011. An
updated query was later conducted to include studies published through August
2012.
In January of 2021, the Renal Mass and Localized Renal Cancer guideline
underwent an additional amendment based on a current literature search. This
Approved by the AUA
Board of Directors April
2021
Authors’ disclosure of po-
tential conflicts of interest
and author/staff contribu-
tions appear at the end of
the article.
© 2021 by the American
Urological Association
American Urological Association (AUA)
RENAL MASS AND LOCALIZED RENAL CANCER:
EVALUATION, MANAGEMENT, AND FOLLOW-UP: AUA
GUIDELINE
Steven Campbell, MD, PhD; Robert G. Uzzo, MD; Mohamad E. Allaf, MD; Jay Todd
Bishoff, MD; Eric B. Bass, MD, MPH; Jeffrey A. Cadeddu, MD; Anthony Chang,
MD; Sam S. Chang, MD; Peter E. Clark, MD; Jonathan A. Coleman, MD; Philipp
Dahm, MD; Brian J. Davis, MD, PhD; Ithaar H. Derweesh, MD; Mireya Diaz, PhD;
Sherri M. Donat , MD; Leo Giambarresi, PhD; Debra A. Gervais, MD; S. Duke
Herrell III, MD; Susan Hilton, MD; Susie L. Hu, MD; Eric Jonasch, MD; Jose A.
Karam, MD; Brian R. Lane, MD, PhD; Bradley C. Leibovich, MD, FACS; Daniel Wei
Lin, MD; Philip M. Pierorazio, MD; Victor Edward Reuter, MD; Lesley Souter, PhD
Copyright © 2021 American Urological Association Education and Research, Inc.®
Panel Nomination
Acknowledgment:
The AUA would like to
acknowledge the following
organizations: College of
American Pathologists
(CAP); Society of Urologic
Oncologists (SUO); Ameri-
can College of Radiology
(ACR); American Society of
Nephrology (ASN); En-
dourological Society; and
the Society of Interventional
Radiology for participation
in the development of this
guideline.

2
American Urological Association (AUA)
literature search retrieved additional studies published between July 2016 to October 2020 using the same Key
Questions and search criteria from the Renal Mass and Localized Renal Cancer guideline. Nineteen studies were
identified from this search to provide data relevant to the management and treatment of Renal Mass. In addition, the
Follow-Up for Clinically Localized Renal Neoplasms guideline published in 2013 was merged with the Renal Mass and
Localized Renal Cancer guideline. Although the systematic search for follow-up interventions was not updated to
2020, the panel members conducted a comprehensive review of all evidence published since the original guideline.
The language of many statements has been refined for clarity. For all evidence-based statements, supporting
studies were identified only in the original systematic review and the evidence strength was not altered.
When sufficient evidence existed, the body of evidence for a particular treatment was assigned a strength rating of A
(high), B (moderate) or C (low) for support of Strong, Moderate, or Conditional Recommendations. In the absence of
sufficient evidence, additional information is provided as Clinical Principles and Expert Opinions.
GUIDELINE STATEMENTS
INITIAL EVALUATION AND DIAGNOSIS
Evaluation
1. In patients with a solid or complex cystic renal mass, clinicians should obtain high quality, multiphase, cross-
sectional abdominal imaging to optimally characterize and clinically stage the renal mass. Characterization of the
renal mass should include assessment of tumor complexity, degree of contrast enhancement (where applicable), and
presence or absence of fat. (Clinical Principle)
2. In patients with suspected renal malignancy, clinicians should obtain a comprehensive metabolic panel, complete
blood count, and urinalysis. Metastatic evaluation should include chest imaging to evaluate for possible thoracic
metastases. (Clinical Principle)
3. For patients with a solid or Bosniak 3/4 complex cystic renal mass, clinicians should assign chronic kidney disease
(CKD) stage based on glomerular filtration rate (GFR) and degree of proteinuria. (Expert Opinion)
Counseling
4. In patients with a solid or Bosniak 3/4 complex cystic renal mass, a urologist should lead the counseling process
and should consider all management strategies. A multidisciplinary team should be included when necessary. (Expert
Opinion)
5. Clinicians should provide counseling that includes current perspectives about tumor biology and a patient-specific
risk assessment inclusive of sex, tumor size/complexity, histology (when obtained), and imaging characteristics. For
cT1a tumors, the low oncologic risk of many small renal masses should be reviewed. (Clinical Principle)
6. During counseling of patients with a solid or Bosniak 3/4 complex cystic renal mass, clinicians must review the
most common and serious urologic and non-urologic morbidities of each treatment pathway and the importance of
patient age, comorbidities/frailty, and life expectancy. (Clinical Principle)
7. Clinicians should review the importance of renal functional recovery related to renal mass management, including
the risks of progressive CKD, potential short- or long-term need for renal replacement therapy, and long-term overall
survival considerations. (Clinical Principle)
8. Clinicians should consider referral to nephrology in patients with a high risk of CKD progression, including those
with estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73m2, confirmed proteinuria, diabetics with
preexisting CKD, or whenever eGFR is expected to be less than 30 mL/min/1.73m2 after intervention. (Expert
Opinion)
9. Clinicians should recommend genetic counseling for any of the following: all patients ≤ 46 years of age with renal
malignancy, those with multifocal or bilateral renal masses, or whenever 1) the personal or family history suggests a
familial renal neoplastic syndrome; 2) there is a first-or second-degree relative with a history of renal malignancy or
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

3
a known clinical or genetic diagnosis of a familial renal neoplastic syndrome (even if kidney cancer has not been
observed); or 3) the patient’s pathology demonstrates histologic findings suggestive of such a syndrome. (Expert
Opinion)
Renal Mass Biopsy (RMB)
10. When considering the utility of RMB, patients should be counseled regarding rationale, positive and negative
predictive values, potential risks and non-diagnostic rates of RMB. (Moderate Recommendation; Evidence Level:
Grade C)
11. Clinicians should consider RMB when a mass is suspected to be hematologic, metastatic, inflammatory, or
infectious. (Clinical Principle)
12. In the setting of a solid renal mass, RMB should be obtained on a utility-based approach whenever it may
influence management. RMB is not required for 1) young or healthy patients who are unwilling to accept the
uncertainties associated with RMB; or 2) older or frail patients who will be managed conservatively independent of
RMB findings. (Expert Opinion)
13. For patients with a solid renal mass who elect RMB, multiple core biopsies should be performed and are preferred
over fine needle aspiration (FNA). (Moderate Recommendation; Evidence Level: Grade C)
MANAGEMENT
Partial Nephrectomy (PN) and Nephron-Sparing Approaches
14. Clinicians should prioritize PN for the management of the cT1a renal mass when intervention is indicated. In this
setting, PN minimizes the risk of CKD or CKD progression and is associated with favorable oncologic outcomes,
including excellent local control. (Moderate Recommendation; Evidence Level: Grade B)
15. Clinicians should prioritize nephron-sparing approaches for patients with solid or Bosniak 3/4 complex cystic
renal masses and an anatomic or functionally solitary kidney, bilateral tumors, known familial RCC, preexisting CKD,
or proteinuria. (Moderate Recommendation; Evidence Level: Grade C)
16. Nephron-sparing approaches should be considered for patients with solid or Bosniak 3/4 complex cystic renal
masses who are young, have multifocal masses, or comorbidities that are likely to impact renal function in the
future, including but not limited to moderate to severe hypertension, diabetes mellitus, recurrent urolithiasis, or
morbid obesity. (Moderate Recommendation; Evidence Level: Grade C)
17. In patients who elect PN, clinicians should prioritize preservation of renal function by optimizing nephron mass
preservation and avoiding prolonged warm ischemia. (Expert Opinion)
18. For patients undergoing PN, clinicians should prioritize negative surgical margins. The extent of normal
parenchyma removed should be determined by surgeon discretion taking into account the clinical situation and
tumor characteristics, including growth pattern, and interface with normal tissue. Tumor enucleation should be
considered in patients with familial RCC, multifocal disease, or severe CKD to optimize parenchymal mass
preservation. (Expert Opinion)
Radical Nephrectomy (RN)
19. Clinicians should consider RN for patients with a solid or Bosniak 3/4 complex cystic renal mass whenever
increased oncologic potential is suggested by tumor size, RMB (if obtained), and/or imaging. (Moderate
Recommendation; Evidence Level: Grade B) In this setting, RN is preferred if all of the following criteria are met: 1)
high tumor complexity and PN would be challenging even in experienced hands; 2) no preexisting CKD or
proteinuria; and 3) normal contralateral kidney and new baseline eGFR will likely be greater than 45 mL/min/1.73m2
even if RN is performed. If all of these criteria are not met, PN should be considered unless there are overriding
concerns about the safety or oncologic efficacy of PN. (Expert Opinion)
Surgical Principles
20. For patients who are undergoing surgical excision of a renal mass with clinically concerning regional
lymphadenopathy, clinicians should perform a lymph node dissection including all clinically positive nodes for staging
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

4
purposes. (Expert Opinion)
21. For patients who are undergoing surgical excision of a renal mass, clinicians should perform adrenalectomy if
imaging and/or intraoperative findings suggest metastasis or direct invasion of the adrenal gland. (Clinical Principle)
22. In patients undergoing surgical excision of a renal mass, a minimally invasive approach should be considered
when it would not compromise oncologic, functional, and perioperative outcomes. (Expert Opinion)
Other Considerations
23. Pathologic evaluation of the adjacent renal parenchyma should be performed and recorded after PN or RN to
assess for possible intrinsic renal disease, particularly for patients with CKD or risk factors for developing CKD.
(Clinical Principle)
24. Clinicians should consider referral to medical oncology whenever there is concern for potential clinical metastasis
or incompletely resected disease (macroscopic positive margin or gross residual disease). Patients with high-risk or
locally advanced, fully resected renal cancers should be counselled about the risks/benefits of adjuvant therapy and
encouraged to participate in adjuvant clinical trials, facilitated by medical oncology consultation when needed.
(Clinical Principle)
Thermal Ablation (TA)
25. Clinicians should consider TA as an alternate approach for the management of cT1a solid renal masses <3 cm in
size. For patients who elect TA, a percutaneous technique is preferred over a surgical approach whenever feasible to
minimize morbidity. (Moderate Recommendation; Evidence Level: Grade C)
26. Both radiofrequency ablation (RFA) and cryoablation may be offered as options for patients who elect TA.
(Conditional Recommendation; Evidence Level: Grade C)
27. A RMB should be performed prior to (preferred) or at the time of ablation to provide pathologic diagnosis and
guide subsequent surveillance. (Expert Opinion)
28. Counseling about TA should include information regarding an increased likelihood of tumor persistence or local
recurrence after primary TA relative to surgical excision, which may be addressed with repeat ablation if further
intervention is elected. (Strong Recommendation; Evidence Level: Grade B)
Active Surveillance (AS)
29. For patients with a solid renal mass < 2cm, or those that are complex but predominantly cystic, clinicians may
elect AS with potential for delayed intervention for initial management. (Conditional Recommendation; Evidence
Level: Grade C)
30. For patients with a solid or Bosniak 3/4 complex cystic renal mass, clinicians should prioritize AS/expectant
management when the anticipated risk of intervention or competing risks of death outweigh the potential oncologic
benefits of active treatment. In asymptomatic patients, the panel recommends periodic clinical surveillance and/or
imaging based on shared decision making. (Clinical Principle)
31. For patients with a solid or Bosniak 3/4 complex cystic renal mass in whom the risk/benefit analysis for
treatment is equivocal and who prefer AS, clinicians should consider RMB (if the mass is solid or has solid
components) for further oncologic risk stratification. Repeat cross-sectional imaging should be obtained
approximately 3-6 months later to assess for interval growth. Periodic clinical/imaging surveillance can then be
based on growth rate and shared decision-making with intervention recommended if substantial interval growth is
observed or if other clinical/imaging findings suggest that the risk/benefit analysis is no longer equivocal or favorable
for continued AS. (Expert Opinion)
32. For patients with a solid or Bosniak 3/4 complex cystic renal mass in whom the anticipated oncologic benefits of
intervention outweigh the risks of treatment and competing risks of death, clinicians should recommend intervention.
AS with potential for delayed intervention may be pursued only if the patient understands and is willing to accept the
associated oncologic risks. In this setting, clinicians should encourage RMB (if the mass is predominantly solid) for
additional risk stratification. If the patient continues to prefer AS, close clinical and cross-sectional imaging
surveillance with periodic reassessment and counseling should be recommended. (Moderate Recommendation;
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

5
Evidence Level: Grade C)
FOLLOW-UP AFTER INTERVENTION
General Principles
33. Clinicians coordinating follow-up for patients who have undergone intervention for a renal mass should discuss
the implications of stage, grade, and histology including the risks of recurrence and possible sequelae of treatment.
Patients with pathologically-proven benign renal masses should undergo occasional clinical evaluation and laboratory
testing for sequelae of treatment but most do not require routine periodic imaging. (Expert Opinion)
34. Patients with treated malignant renal masses should undergo periodic medical history, physical examination,
laboratory studies, and imaging directed at detecting signs and symptoms of metastatic spread and/or local
recurrence as well as evaluation for possible sequelae of treatment. (Clinical Principle)
35. Patients with treated malignant renal masses should have periodic laboratory testing including serum creatinine,
eGFR, and urinalysis. Other laboratory evaluations (e.g., complete blood count, lactate dehydrogenase, liver function
tests, alkaline phosphatase and calcium level) may be obtained at the discretion of the clinician or if advanced
disease is suspected. (Expert Opinion)
36. Patients undergoing follow-up for treated renal masses with progressive renal insufficiency or proteinuria should
be referred to nephrology. (Expert Opinion)
37. Patients undergoing follow-up for treated malignant renal masses should only undergo bone scan if one or more
of the following is present: clinical symptoms such as bone pain, elevated alkaline phosphatase, or radiographic
findings suggestive of a bony neoplasm. (Moderate Recommendation; Evidence Level: Grade C)
38. Patients undergoing follow-up for treated malignant renal masses with acute neurological signs or symptoms
should undergo prompt magnetic resonance imaging (MRI) or computed tomography (CT) scanning of the brain and/
or spine. (Strong Recommendation; Evidence Level: Grade A)
39. For patients undergoing follow-up for treated malignant renal masses, additional site-specific imaging can be
ordered as warranted by clinical symptoms suggestive of recurrence or metastatic spread. Positron emission
tomography (PET) scan should not be obtained routinely but may be considered selectively. (Moderate
Recommendation; Evidence Level: Grade C)
40. Patients with findings suggestive of metastatic renal malignancy should be evaluated to define the extent of
disease and referred to medical oncology. Surgical resection or ablative therapies should be considered in select
patients with isolated or oligo-metastatic disease. (Expert Opinion)
41. Patients with findings suggesting a new renal primary or local recurrence of renal malignancy should undergo
metastatic evaluation including chest and abdominal imaging. If the new primary or recurrence is isolated to the
ipsilateral kidney and/or retroperitoneum, a urologist should be involved in the decision-making process, and surgical
resection or ablative therapies may be considered. (Expert Opinion)
Follow-up After Surgery
42. Clinicians should classify patients who have been managed with surgery (PN or RN) for a malignant renal mass
into one of the following risk groups for follow-up:
If final microscopic surgical margins are positive for cancer, the risk category should be considered at least one level
higher, and increased clinical vigilance should be exercised. (Expert Opinion)
Low Risk (LR): pT1 and Grade 1/2
Intermediate Risk (IR): pT1 and Grade 3/4, or pT2 any Grade
High Risk (HR): pT3 any Grade
Very High Risk (VHR): pT4 or pN1, or sarcomatoid/rhabdoid dedifferentia-
tion, or macroscopic positive margin
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

6
43. Patients managed with surgery (PN or RN) for a renal malignancy should undergo abdominal imaging according
to Table 1, with CT or MRI pre- and post-intravenous contrast preferred. (Moderate Recommendation; Evidence
Strength: Grade C). After 2 years, abdominal ultrasound (US) alternating with cross-sectional imaging may be
considered in the LR and IR groups at physician discretion. After 5 years, informed/shared decision-making should
dictate further abdominal imaging. (Expert Opinion)
44. Patients managed with surgery (PN or RN) for a renal malignancy should undergo chest imaging (chest x-ray
[CXR] for LR and IR; CT chest preferred for HR and VHR) according to Table 1. (Moderate Recommendation;
Evidence Strength: Grade C). After 5 years, informed/shared decision-making discussion should dictate further chest
imaging and CXR may be utilized instead of chest CT for HR and VHR (Expert Opinion)
Table 1: Recommended follow-up schedule after surgery for renal cancer (in months)*
*Follow-up timeline is approximate and allows flexibility to accommodate reasonable patient, caregiver, and
institutional needs. Each follow-up visit should include relevant history, physical examination, laboratory testing, and
abdominal and chest imaging. Overall, 30% of renal cancer recurrences after surgery are diagnosed beyond 60
months.
1
Informed/shared decision-making should guide surveillance decisions beyond 60 months.
Follow-up After TA
45. Patients undergoing ablative procedures with biopsy that confirmed malignancy or was non-diagnostic should
undergo pre- and post-contrast cross-sectional abdominal imaging within 6 months (if not contraindicated).
Subsequent follow-up should be according to the recommendations for the IR postoperative protocol (Table 1).
(Expert Opinion)
Risk 3 6 9 12 18 24 30 36 48 60 72-84 96-120
LR x x x x x x
IR x x x x x x x x
HR x x x x x x x x x x
VHR x x x x x x x x x x x x
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

7
INTRODUCTION
PURPOSE
This AUA Guidelines focuses on the evaluation and
management of clinically localized sporadic renal
masses suspicious for RCC in adults, including solid
enhancing renal tumors and Bosniak 3 and 4 complex
cystic renal masses. Some patients with clinically
localized renal masses may present with findings
suggesting aggressive tumor biology or may be
upstaged on exploration or final pathology.
Management considerations pertinent to the urologist in
such patients will also be discussed. The follow-up of
renal cancer patients after intervention is also
addressed including recommendations for periodic
clinical follow-up and abdominal and chest imaging.
Practice patterns regarding such tumors vary
considerably and the literature regarding evaluation,
management, and surveillance has been rapidly
evolving. Notable examples include controversies about
the role of RMB and concerns about overutilization of
RN.
METHODOLOGY
Systematic Review of Renal Mass.
The systematic review utilized in the creation of this
guideline was completed in part through the AHRQ and
through additional supplementation that further
addressed additional key questions and more recently
published literature. A research librarian experienced in
conducting literature searches for comparative
effectiveness reviews searched in MEDLINE®, Embase
®, the Cochrane Library, the Database of Abstracts of
Reviews of Effects, the Health Technology Assessment
Database, and the UK National Health Service Economic
Evaluation database to capture both published and gray
literature published from January 1, 1997 through May
1, 2015. A supplemental search was conducted adding
additional literature published through August 2015,
and a final update search was conducted through July
2016.
Systematic Review Follow-up Renal Cancer.
A systematic review was conducted to identify
published articles relevant to key questions specified by
the Panel related to kidney neoplasms and their follow-
up (imaging, renal function, markers, biopsy, and
prognosis). This search covered articles in English
published between January 1999 and 2011. An updated
query was later conducted to include studies published
through August 2012. Study designs consisting of
clinical trials (randomized or not), observational studies
(cohort, case-control, case series) and systematic
reviews were included. All other study types were
excluded. Studies with full-text publication available
were included, but studies in abstract form only were
excluded.
Combination of Guidelines
In January of 2021, the Renal Mass and Localized Renal
Cancer guideline underwent an additional amendment
based on current literature. The updated literature
search retrieved additional studies published between
July 2016 to October 2020 using the same search
strategy from the Renal Mass and Localized Renal
Cancer guideline. Following study selection using the
original PICO criteria, as reporting data relevant to the
management and treatment of Renal Mass. In addition,
the Follow-Up for Clinically Localized Renal Neoplasms
guideline published in 2013 was merged with the Renal
Mass and Localized Renal Cancer guideline. Although
the systematic search for follow-up interventions was
not updated to 2020, the panel members conducted a
comprehensive review of all evidence published since
the original guideline. The language of many
statements has been refined for clarity. For all
evidence-based statements, supporting studies were
identified only in the original systematic review and the
evidence strength was not altered.
Assessment of Risk-of-Bias of Individual Studies.
Citations identified by the systematic search were
screened independently by two reviewers using
predefined PICO criteria. One reviewer completed data
abstraction and a second reviewer checked abstraction
for accuracy. Two reviewers independently assessed
risk of bias for individual studies. The Cochrane
Collaboration’s tool was used for assessing the risk of
bias of randomized controlled trials (RCTs).
2
For
nonrandomized studies of treatment interventions, the
reviewers used the Risk of Bias in Non-Randomized
Studies – of Intervention (ROBINS-I). For diagnostic
studies, we used the quality assessment tool for
diagnostic accuracy studies (QUADAS -2).
3,4
Differences
between reviewers were resolved through consensus.
Determination of Evidence Strength.
The categorization of evidence strength is conceptually
distinct from the quality of individual studies. Evidence
strength refers to the body of evidence available for a
particular question and includes not only individual
study quality but consideration of study design,
consistency of findings across studies, adequacy of
sample sizes, and generalizability of samples, settings,
and treatments for the purposes of the guideline. The
AUA categorizes body of evidence strength as Grade A
(well-conducted and highly-generalizable RCTs or
exceptionally strong observational studies with
consistent findings), Grade B (RCTs with some
weaknesses of procedure or generalizability or
moderately strong observational studies with consistent
findings), or Grade C (RCTs with serious deficiencies of
procedure or generalizability or extremely small sample
sizes or observational studies that are inconsistent,
have small sample sizes, or have other problems that
potentially confound interpretation of data). By
definition, Grade A evidence is evidence about which
the Panel has a high level of certainty, Grade B
evidence is evidence about which the Panel has a
moderate level of certainty, and Grade C evidence is
evidence about which the Panel has a low level of
certainty.
5
AUA Nomenclature: Linking Statement Type to
Evidence Strength.
The AUA nomenclature system explicitly links statement
type to body of evidence strength, level of certainty,
magnitude of benefit or risk/burdens, and the Panel’s
judgment regarding the balance between benefits and
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

8
risks/burdens (Table 2). Strong Recommendations are
directive statements that an action should (benefits
outweigh risks/burdens) or should not (risks/burdens
outweigh benefits) be undertaken because net benefit
or net harm is substantial. Moderate Recommendations
are directive statements that an action should (benefits
outweigh risks/burdens) or should not (risks/burdens
outweigh benefits) be undertaken because net benefit
or net harm is moderate. Conditional Recommendations
are non-directive statements used when the evidence
indicates that there is no apparent net benefit or harm
or when the balance between benefits and risks/burden
is unclear. All three statement types may be supported
by any body of evidence strength grade. Body of
evidence strength Grade A in support of a Strong or
Moderate Recommendation indicates that the statement
can be applied to most patients in most circumstances
and that future research is unlikely to change
confidence. Body of evidence strength Grade B in
support of a Strong or Moderate Recommendation
indicates that the statement can be applied to most
patients in most circumstances but that better evidence
could change confidence. Body of evidence strength
Grade C in support of a Strong or Moderate
Recommendation indicates that the statement can be
applied to most patients in most circumstances but that
better evidence is likely to change confidence. Body of
evidence strength Grade C is only rarely used in
support of a Strong Recommendation. Conditional
Recommendations can also be supported by any
evidence strength. When body of evidence strength is
Grade A, the statement indicates that benefits and
risks/burdens appear balanced, the best action depends
on patient circumstances, and future research is
unlikely to change confidence. When body of evidence
strength Grade B is used, benefits and risks/burdens
appear balanced, the best action also depends on
individual patient circumstances and better evidence
could change confidence. When body of evidence
strength Grade C is used, there is uncertainty regarding
the balance between benefits and risks/burdens,
alternative strategies may be equally reasonable, and
better evidence is likely to change confidence.
Where gaps in the evidence existed, the Panel provides
guidance in the form of Clinical Principles or Expert
Opinion with consensus achieved using a modified
Delphi technique if differences of opinion emerged.
6
A
Clinical Principle is a statement about a component of
clinical care that is widely agreed upon by urologists or
other clinicians for which there may or may not be
evidence in the medical literature. Expert Opinion refers
to a statement, achieved by consensus of the Panel,
that is based on members' clinical training, experience,
knowledge, and judgment for which there is no
evidence.
Process
The Renal Mass and Localized Renal Cancer Panel was
created in 2014 by the American Urological Association
Education and Research, Inc. (AUA). The Practice
Guidelines Committee (PGC) of the AUA selected the
Panel Chair who in turn appointed the Vice Chair. In a
collaborative process, additional Panel members,
including additional members of the College of
American Pathologists (CAP), Society of Urologic
Oncology (SUO), American College of Radiology (ACR),
American Society of Nephrology (ASN), Endourological
Society, and Society of Interventional Radiology (SIR)
with specific expertise in this area, were then
nominated and approved by the PGC. The AUA
conducted a thorough peer review process. The draft
guidelines document was distributed to 124 peer
reviewers, 54 of which submitted comments. The Panel
reviewed and discussed all submitted comments and
revised the draft as needed. Once finalized, the
guideline was submitted for approval to the PGC and
Science and Quality Council. Then it was submitted to
the AUA, CAP, SUO, ACR, ASN, Endourological Society,
and SIR Board of Directors for final approval. Panel
members received no remuneration for their work.
A Renal Mass amendment panel consisting of five
members was created in August 2020 to conduct an
update to the Renal Mass and Localized Renal Cancer
guideline. They were also tasked with integrating the
Follow-Up for Clinically Localized Renal Neoplasms
guideline from 2013 into the Renal Mass and Localized
Renal Cancer guideline from 2017 to create one
cohesive document on management and follow-up on
renal mass. The panel consisted of members from both
the Follow-Up for Clinically Localized Renal Neoplasms
guideline and the Renal Mass and Localized Renal
Cancer guideline with an additional new member who
has not previously served on one of these panels. The
AUA conducted a thorough peer review process. The
draft guidelines document was distributed to 75 peer
reviewers, 21 of which submitted comments. The Panel
reviewed and discussed all submitted comments and
revised the draft as needed. Once finalized, the
guideline was submitted for approval to the PGC and
Science and Quality Council and the AUA Board of
Directors for final approval. Panel members received no
remuneration for their work.
BACKGROUND
Renal masses are a biologically heterogeneous group of
tumors ranging from benign masses to cancers that can
be indolent or aggressive.
7,8
The true incidence of renal
masses (including benign masses) is unknown.
However, benign masses comprise approximately 15-20
percent of surgically resected tumors < 4 cm and allow
estimations of benign incidence based on kidney cancer
statistics.
7,9,10
The vast majority (greater than 90%) of
kidney cancers in the United States are renal cortical
tumors known as RCC.
Epidemiology: United States
It is estimated there will be over 73,000 new cases of
kidney cancer in the United States in 2020.
11,12
The
incidence of kidney cancer has been increasing steadily
since the 1970’s in part due to more prevalent use of
axial imaging (CT and MRI).
13
In the United States,
over the past decade, the incidence of kidney cancer
continues to increase but at a much smaller increment,
approximately 1% per year. The greatest increase in
incidence has been in small, clinically localized renal
masses which now represent at least 40 percent of
incident tumors.
14,15
The overall survival rate for all stages of renal cancer is
approximately 74%, leaving an estimated 400,000
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

9
TABLE 2: AUA Nomenclature Linking Statement Type to Level of Certainty, Magnitude of Benefit or
Risk/Burden, and Body of Evidence Strength
Evidence Strength A
(High Certainty)
Evidence Strength B
(Moderate Certainty)
Evidence Strength C
(Low Certainty)
Strong
Recommendation
(Net benefit or harm sub-
stantial)
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is substantial
Applies to most patients
in most circumstances
and future research is
unlikely to change confi-
dence
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is substantial
Applies to most patients
in most circumstances but
better evidence could
change confidence
Benefits > Risks/Burdens (or
vice versa)
Net benefit (or net harm)
appears substantial
Applies to most patients in
most circumstances but bet-
ter evidence is likely to
change confidence
(rarely used to support a
Strong Recommendation)
Moderate
Recommendation
(Net benefit or harm
moderate)
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is moderate
Applies to most patients
in most circumstances
and future research is
unlikely to change confi-
dence
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is moderate
Applies to most patients
in most circumstances but
better evidence could
change confidence
Benefits > Risks/Burdens (or
vice versa)
Net benefit (or net harm)
appears moderate
Applies to most patients in
most circumstances but bet-
ter evidence is likely to
change confidence
Conditional
Recommendation
(No apparent net benefit
or harm)
Benefits = Risks/Burdens
Best action depends on
individual patient circum-
stances
Future research unlikely
to change confidence
Benefits = Risks/Burdens
Best action appears to
depend on individual pa-
tient circumstances
Better evidence could
change confidence
Balance between Benefits &
Risks/Burdens unclear
Alternative strategies may
be equally reasonable
Better evidence likely to
change confidence
Clinical Principle
A statement about a component of clinical care that is widely agreed upon by urolo-
gists or other clinicians for which there may or may not be evidence in the medical
literature
Expert Opinion
A statement, achieved by consensus of the Panel, that is based on members' clinical
training, experience, knowledge, and judgment for which there is for which there may
or may not be evidence in the medical literature
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

10
kidney cancer survivors in the United States as of
2013.
11
However, approximately 14,800 men and
women will die of kidney cancer in 2020.
10
The
mortality from kidney cancer has been steadily
decreasing, approximately 1% per year, since 2004.
16,17
Reasons for this decrease are multifactorial.
Kidney cancer is more common in men than women,
and more common in African Americans, American
Indian and Alaska Native populations than
Caucasians.
18
The median age at diagnosis is 64 years
old, although kidney cancer can present at any age.
19
Epidemiology: Global and International
Considerations
Over 300,000 men and women are diagnosed with
kidney cancer around the world each year and
approximately 150,000 patients will die of disease.
20
The incidence of kidney cancer varies dramatically
around the world with the developed countries having
the highest rates.
21
Incidence rates have increased in
both sexes and are most notable in the elderly
population (greater than 75 years of age). Mortality
rates have been stable in most countries but have been
decreasing by 1 to 3 percent in Western and Northern
Europe, the United States, and Australia. The improved
mortality globally and in the US is attributed to
decreased smoking rates, improved therapies, and
access to medical care. The decrease in mortality has
been faster in women than in men and overall mortality
rates remain higher in men than women.
Etiology
There are a number of established and putative risk
factors for RCC. Smoking is a well-established risk
factor, accounting for 20 percent of incident cases and
increasing the risk of RCC by 50 percent in men and 20
percent in women., Obesity is associated with 30% of
incident cases of RCC and each 5 kg/m2 increase in
body mass index increases the risk of RCC by 24
percent in men and 34 percent in women.
23-25
Interestingly, an “obesity paradox” exists in kidney
cancer – where obese patients are more likely to
develop RCC, but these tumors are more likely to be
low-grade, early stage tumors.
25-27
Hypertension is also
associated with increased risk of RCC.
23,28,29
The role of
CKD as a risk factor is controversial; however, patients
on maintenance dialysis are also reported to have an
increased risk of RCC.
30
The data regarding
environmental and occupational exposures are
inconsistent with the exception of chlorinated
solvents.
23,31
Moderate alcohol intake,
32,33
consumption of fruits and
(cruciferous) vegetables,
2,3,34,35
and a diet rich in fatty
fish
36
are believed to reduce the risk of RCC. Other
studies suggest that non-steroidal anti-inflammatory
agents and dietary factors do not play a role in the
etiology of RCC.
5,23,37
Hereditary and Familial RCC
Family history is associated with an increased risk of
RCC and a number of familial RCC syndromes are now
well-established, accounting for approximately 4-6% of
cases of RCC overall.
38
These syndromes include von
Hippel-Lindau (VHL), hereditary papillary renal
carcinoma (HPRC), Birt Hogg-Dubé (BHD), hereditary
leiomyomatosis RCC (HLRCC), succinate dehydrogenase
deficiency RCC, tuberous sclerosis, BAP-1 tumor
predisposition syndrome, and PTEN hamartoma tumor
syndrome (Cowden syndrome). Most of these
syndromes have associated tumors or benign findings
in other organ systems. RCC in these syndromes tends
to be earlier in onset and multifocal and management
should prioritize nephron-sparing approaches, including
tumor enucleation when feasible to optimize
preservation of parenchymal mass. For most of these
syndromes, tumors can be observed if less than 3 cm
as the risk of metastases remains low in this setting.
39
HLRCC and succinate dehydrogenase deficiency RCC
are the exception as tumors in these syndromes are
often very aggressive and a proactive approach to
evaluation and management should be pursued.
Genetic counseling should also be strongly
recommended for patients suspected of having familial
RCC, as it may allow for more intensive evaluation of
the patient for RCC and associated manifestations and
identification of blood relatives that may be at
syndromic risk.
Major Pathological Subtypes
Renal tumors are classified based on cell of origin and
morphologic appearance with renal adenocarcinoma
being the most common malignant tumor. Major sub-
classifications of RCC include clear cell, papillary,
chromophobe, collecting duct and unclassified RCC.
40
A
number of uncommon or rare subtypes exist including
but not limited to acquired cystic disease-associated
RCC, clear cell (tubulo) papillary, and renal medullary
carcinoma, which is an aggressive variant typically seen
in patients with sickle cell trait. The most common
benign tumors of the kidney include oncocytoma and
angiomyolipoma (AML). An abbreviated version of the
2016 World Health Organization classification of renal
neoplasms is detailed in Table 3.
41
Presentation and Diagnosis
Presentation
The “classic triad” of symptoms associated with a
malignant renal mass include hematuria, flank pain and
abdominal mass. Symptoms associated with RCC are
often a result of local tumor growth, hemorrhage,
paraneoplastic symptoms, or metastatic disease and
are uncommon in patients with clinically localized
disease. In fact, less than 5 percent of patients in
contemporary series present with these symptoms and
greater than 50 percent of renal masses are diagnosed
incidentally during an evaluation for unrelated signs or
symptoms.
42,43
Diagnosis
Physical examination has a limited role in the diagnosis
of clinically localized disease. However, physical
examination may have value in distinguishing the signs
and symptoms of advanced disease. For instance,
paraneoplastic syndromes (i.e. hypertension,
polycythemia, hypercalcemia) are present in
approximately 10-20 percent of patients with
metastatic RCC.
6,7,44
Importantly, physical examination
of patients with localized disease may occasionally
reveal unsuspected adenopathy, varicocele or medical
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

11
conditions that influence management decisions
including body habitus, prior abdominal scars, stigmata
of CKD, etc. In addition, careful physical examination
may also reveal findings suggestive of familial disease,
such as dermatologic lesions.
TABLE 3. Modified 2016 World Health
Organization classification of renal neoplasms
with focus on adult neoplasms.
41
Renal cell tumors
Clear cell RCC
Multilocular cystic renal neoplasm of low malignant
potential
Papillary RCC
HLRCC
Chromophobe RCC
Collecting duct carcinoma
Renal medullary carcinoma
MiT Family translocation carcinomas
Succinate dehydrogenase (SDH) deficient RCC
Mucinous tubular and spindle cell carcinoma
Tubulocystic RCC
Acquired cystic disease associated RCC
Clear cell papillary RCC
RCC, unclassified
Benign renal tumors
Papillary adenoma
Oncocytoma
AML
Metanephric adenoma and other metanephric tu-
mors
Adult cystic nephroma
Mixed epithelial stromal tumors
Juxtaglomerular cell tumor
Mesenchymal tumors
Leiomyosarcoma (including renal vein) and other
sarcomas
Leiomyoma and other benign mesenchymal tumors
Others
Adult Wilms tumor
Primitive neuroectodermal tumor
Metastatic tumors, lymphoma, leukemia
Laboratory Evaluation
There are no biomarkers or routine laboratory tests
used to diagnose renal malignancies. As such,
laboratory tests are useful in the assessment of renal
function (GFR) and for completeness of metastatic
evaluation. Routine laboratory tests for renal mass
evaluation include complete metabolic panel, complete
blood count, and urinalysis.
Imaging Techniques
Pre and post contrast-enhanced axial imaging, either
CT or MRI, is the ideal imaging technique for the
diagnosis and staging of clinically localized renal
masses. Masses initially diagnosed by US or
intravenous pyelography should be confirmed with pre/
post contrast-enhanced imaging. Depending on tumor
size, 20 to 30 percent of clinically localized renal
masses may be benign.
7,10
Patient and tumor
characteristics can indicate populations more or less
likely to harbor benign or malignant disease. For
instance, women with smaller tumors have a higher
likelihood of having benign tumors.
9,45,46
However, with
the exception of fat-containing AML, none of the current
imaging modalities can reliably distinguish between
benign and malignant tumors or between indolent and
aggressive tumor biology.
Contrast-enhanced abdominal imaging (CT or MRI) best
characterizes the mass, provides information regarding
renal morphology (of the affected and unaffected
kidney), assesses extrarenal tumor spread (venous
invasion or regional lymphadenopathy) and evaluates
the adrenal glands and other abdominal organs for
visceral metastases. Based on the most recent
consensus statement from the ACR and the National
Kidney Foundation, patients with acute kidney injury or
CKD and GFR less than 30 mL/min/1.73m
2
who are not
undergoing renal replacement therapy should receive
intravenous normal saline prophylaxis prior to receiving
iodinated contrast media.
47
Patients with GFR of 30-44
mL/min/1.73m
2
may be considered for intravenous fluid
prophylaxis per individual physician discretion based on
the patient’s risk factor for renal injury. However, MRI
with second generation gadolinium-based intravenous
contrast is now a safer option in many patients with
severe CKD, as outlined below.
The association of gadolinium-based MRI contrast
agents with the development of nephrogenic systemic
fibrosis – a devastating and potentially fatal condition
has been a concern for many years. More recently,
however, with newer group II and III gadolinium-based
contrast media the risk is felt to be lower than
previously perceived. The most recent consensus
statement from the ACR and the National Kidney
Foundation suggests that such agents can be given to
patients with a GFR under 30 mL/min/1.73m
2
.
48
A
recent systematic review of the risks of NSF in patients
with CKD 4 and 5 noted that the risks of NSF using
group II gadolinium-based agents was less than 0.07%.
Current ACR guidelines on the use of contrast media
state that patients need not be screened for renal
function prior to receiving group II gadolinium-based
agents. Non-contrast CT, MRI (with diffusion weighted
images) and US (with Doppler and with or without
microbubbles) can also be used to characterize renal
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
12
masses in patients who cannot receive conventional
intravenous contrast.
47
In general, solid renal masses that enhance greater
than 15-20 HU with intravenous contrast and do not
exhibit fat density should be considered suspicious for
RCC. Approximately 5-10% of AML’s are fat poor and
difficult to identify on imaging. Fat poor AML’s often
demonstrate suggestive features such as high
attenuation on unenhanced CT, homogeneous
enhancement on CT, or hypointensity on T2-weighted
MR, but the diagnosis remains difficult. Complex cystic
renal masses that have thickened irregular walls or
septa in which measurable enhancement is present are
classified as Bosniak 3. Approximately 50% of such
lesions prove to be malignant on final pathology.
Bosniak 4 complex cystic lesions are very suspicious for
malignancy as they contain enhancing nodular soft
tissue components and about 75-90% of such lesions
prove to be RCC on final pathology. This guideline
focuses primarily on the evaluation and management of
clinically localized sporadic renal masses suspicious for
RCC in adults, including solid enhancing renal tumors
and Bosniak 3 and 4 cystic renal masses.
In patients with RCC or suspicion of RCC, complete
staging is typically finalized with chest radiography
(CXR) or chest CT. Chest CT scan should be obtained
selectively, primarily for patients with pulmonary
symptoms or abnormal CXR, or for patients with high-
risk disease.
49,50
Bone scans should be reserved
primarily for patients with bone pain or elevated
alkaline phosphatase and brain imaging for those with
neurologic symptoms.
51-53
PET scan has a very limited
role in the routine evaluation or staging of RCC.
Renal Mass Biopsy
RMB currently has an adjunctive role in the diagnosis
and risk stratification of patients with renal masses
suspicious for renal cancer. Biopsy, or FNA, was
traditionally reserved for patients suspected of having
metastasis of another primary to the kidney, abscess,
or lymphoma, or when needed to establish a pathologic
diagnosis of RCC in occasional patients presenting with
disseminated metastases or unresectable primary
tumors. The role of RMB for clinically localized RCC has
evolved considerably over the past few decades with
substantial variance in practice patterns.
Tumor Characteristics
Staging
Kidney cancer is staged both clinically and
pathologically using the staging system outlined by the
American Joint Committee on Cancer (AJCC), also
known as the tumor node metastases (TNM)
classification.
54
The AJCC TNM Staging System for
Kidney Cancer is detailed in Table 4. Stage I and II
tumors include cancers of any size that are confined to
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Primary Tumor (T)
TX Primary tumor cannot be assessed.
T0 No evidence of primary tumor.
T1 Tumor ≤7 cm in greatest dimension, limited to the kidney.
T1a Tumor ≤4 cm in greatest dimension, limited to the kidney.
T1b Tumor >4 cm but not >7 cm in greatest dimension, limited to the kidney.
T2 Tumor >7 cm in greatest dimension, limited to the kidney.
T2a Tumor >7 cm but ≤10 cm in greatest dimension, limited to the kidney.
T2b Tumor >10 cm, limited to the kidney.
T3 Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland
and not beyond Gerota’s fascia.
T3a Tumor extends into the renal vein or its segmental branches, or invades the pelvicaliceal system,
or invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia.
T3b Tumor grossly extends into the vena cava below the diaphragm.
T3c Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena
cava.
T4 Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal
gland).
Regional Lymph Nodes (N)
NX Regional lymph nodes cannot be assessed.
N0 No regional lymph node metastasis.
N1 Metastases in regional lymph node(s).
Distant Metastasis (M)
M0 No distant metastasis.
M1 Distant metastasis.
TABLE 4. The AJCC TNM Staging System for Kidney Cancer.
41
Primary Tumor (T), Regional
Lymph Nodes (N) and Distant Metastases (M) are detailed in Table 4A; The Anatomic Stage/
Prognostic Groups are detailed in Table 4B.

13
the kidney. This guideline statement identifies patients
with renal masses suspicious for clinical stage I and II
RCC, recognizing that a certain number of patients will
be upstaged. Stage III tumors are either locally
invasive (T3) or have involved lymph nodes (N1). Stage
IV tumors have spread beyond the kidney into adjacent
organs by direct invasion (T4) or distant metastases
(M1). Prognosis is best predicted by stage with cancer-
specific survival rates that approximate 85-90% for
clinically localized (Stage I and II) RCC.
Grading
Historically, a number of grading systems existed and
evolved to describe tumor differentiation, cytologic
aggressiveness, and prognosis of RCC based on nuclear
size and irregularity. In 1982, the Fuhrman Grading
system was described and became the most widely
used grading system for RCC.
55
In 2012, the
International Society of Urological Pathology (ISUP)
Grading System for RCC was proposed and was
updated in 2016.
41,56
The ISUP Grading System
incorporates aspects of the Fuhrman Grading system
but includes more objective criteria for nuclear
characteristics. In addition, sarcomatoid and rhabdoid
tumors, tumors with giant cells, and tumors with
extreme nuclear pleomorphism are included within
grade 4 tumors. Chromophobe RCC is no longer graded
in the ISUP system. In general, higher grade is
associated with larger tumor size and more aggressive
tumors.
57,58
Other Prognostic Indicators and Nomograms
Other factors for prognostic consideration include tumor
size, necrosis, sarcomatoid features, collecting system
invasion, patient symptoms, signs of paraneoplastic
syndromes, and performance status. Tumor size is
important for risk stratification regarding the likelihood
of malignancy and more aggressive pathology.
7,10,46
Various algorithms including the UCLA Integrated
Staging System (UISS),
59,60
Stage, Size, Grade and
Necrosis (SSIGN) score,
61-63
and other nomograms
8,9,64
incorporate a variety of pathological and patient
characteristics in an effort to improve prognostication.
Other Clinical and Biological Indicators
A number of molecular studies and markers have been
proposed for diagnostic and prognostic purposes in
RCC. The AHRQ Systematic Review identified a number
of biomarkers and laboratory tests that may have
diagnostic or prognostic utility in the renal cancer
literature.
65
However, these studies were often
univariable in design and therefore excluded from
analysis due to a failure to include clinical variables or
suboptimal methodology to validate the ultimate value
of the tests. Therefore, the AHRQ report identified
clinical and biological indicators as a major research
gap in the renal cancer literature.
66
Of note, urine aquaporin-1 and perilipin-2 were
identified as emerging biomarkers with potential for the
diagnosis of RCC.
10,11,67,68
Carbonic anhydrase-9 (CAIX)
expression is governed by the transcription factor
hypoxia-inducible factor-1α (HIF-1α), a well-known
component of the VHL pathway of clear cell RCC.
69
While CAIX expression on primary tumors is a
prognostic factor, especially in patients with metastatic
RCC, high and homogenous levels of CAIX expression
prevent risk stratification and clinical utility beyond the
established clinical predictors of aggressive, clear cell
RCC.
70
Serum tests including C-reactive protein and
platelet count may have prognostic roles, but further
investigation is needed. New imaging modalities,
including molecular imaging techniques using CAIX
71-73
or 99m technetium-sestamibi
74
single photon emission
CT, may help to better differentiate between malignant
and benign pathology. However, most markers and
imaging modalities in this domain are best
characterized as investigational.
Overview of Treatment Alternatives
A number of strategies exist for the management of
sporadic renal masses suspicious for clinically localized
renal cancer. Four strategies are considered standards
of care and include AS, RN, PN, and TA.
Active Surveillance (AS)
A growing body of literature exists regarding AS for
patients with clinically localized small renal masses
(cT1a, ≤4cm). A number of retrospective studies and
meta-analyses evaluate the safety of AS and quote the
risk of metastatic progression while on AS to be less
than 2 percent in well selected patients over the initial
3 years of AS.
75-77
Two large prospective AS programs
have been initiated that follow patients with serial
imaging, and both report slow growth rates and
extremely low rates of metastatic progression, albeit
with relatively short follow-up.
78-80
Both programs
screen patients with an initial metastatic evaluation
including serum laboratory evaluation and chest
imaging. Patients are then evaluated every 3-6 months
for two years and with extended imaging intervals
beyond that. Rates of biopsy are variable with one
group utilizing RMB in greater than 50 percent of the
cohort and the other using biopsy in less than 10
percent of its patients. Further data with longer follow-
up from these cohorts will help to inform the utility of
AS in the small renal mass population, and should allow
for more intelligent patient selection for AS. Of note,
the Delayed Intervention and Surveillance for Small
Renal Masses (DISSRM) Registry prospectively
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
TABLE 4B.
Stage T N M
I T1 N0 M0
II T2 N0 M0
III T1 or T2 N1 M0
T3 N0 or N1 M0
IV T4 Any N M0
Any T Any N M1

14
catalogues a contemporaneous cohort of patients
undergoing AS and primary intervention and will offer
data regarding comparative effectiveness.
80
Radical Nephrectomy (RN)
RN was the mainstay of therapy for all renal masses for
many decades. Historically, RN included the removal of
the entire kidney including Gerota's/Zuckerkandel's
fascia, regional lymph nodes and the adrenal gland. RN
can be performed through an open incision or via
minimally-invasive approaches (laparoscopic or
robotic). Cancer-specific survival associated with RN is
excellent; however, recent controversies regarding RN
include its negative impact on renal function and
historical overutilization for the management of stage I,
especially T1a, tumors.
Partial Nephrectomy (PN)
PN is widely accepted as a nephron-sparing approach to
the management of clinically localized RCC. Initially
underutilized and predominantly performed in large
academic centers,
12,13,81
the management of clinically
localized renal masses by PN has expanded with
implementation of guideline statements and the
expansion of robotic technology.
14,15,82
PN can be
performed through an open incision or via a minimally
invasive approach, although the robotic approach has
largely supplanted laparoscopic surgery as the
preferred minimally invasive approach.
83
The benefit of
PN lies in the potential to preserve renal function but
this is counterbalanced by an increased risk of urologic
complications, although most are manageable and
typically associated with good outcomes. Recent
controversies surround modifiable and non-modifiable
factors during surgery to improve renal functional
outcomes, including parenchymal volume preservation,
warm versus cold ischemia, and duration of ischemia.
Thermal Ablation (TA)
TA techniques were developed in an effort to improve
patient procedural tolerance and reduce the potential
for complications from PN, while still preserving renal
function. A multitude of techniques/technologies have
been investigated to ablate renal tumors; however, RFA
and cryoablation have been most widely investigated
and integrated into clinical practice. While the
superiority of RFA or cryoablation remains
controversial, it is generally accepted that oncologic
outcomes are similar for both approaches.
84-86
TA has
traditionally been performed through a variety of
approaches, including open, laparoscopic, and
percutaneous. Concerns with the TA literature included
relatively limited follow-up, lack of pre and post
treatment biopsy to define malignancy and efficacy,
and increased local recurrence rates relative to surgical
excision. The latter require a longer period of
surveillance (5 years) with cross-sectional imaging to
monitor for late local recurrences.
Investigational Modalities
Other technologies including high-intensity focused US
(HIFU), radiosurgery, microwave therapy, pulsed
cavitational US, and laser thermal therapy remain
investigational at this time.
Follow-up After Intervention
The prognosis of patients treated with surgery or
thermal ablation for kidney cancer is primarily
determined by tumor stage, with tumor size, grade,
histology, with a variety of other contributing
factors.
61,62,87-93
Several algorithms and prognostic
models have been published, yet a recent analysis of
outcomes from a phase III randomized adjuvant clinical
trial suggested that these models only marginally
outperformed stage alone.
94
Current surveillance and survivorship strategies for
patients with RCC have incorporated clinical history,
physical examination, relevant laboratory testing, and
abdominal and chest imaging.
95,181,387
This allows for
assessment of potential complications or sequelae of
intervention, functional recovery, and evaluation for
common sites of recurrence, including those in the
lungs, liver, adrenal glands, and other retroperitoneal
sites. Cross-sectional imaging is generally preferred,
particularly for high-risk patients, while abdominal
ultrasound or chest radiography can be considered in
lower-risk patients or as a potential alternative during
long-term surveillance. Approximately thirty-percent of
recurrences have been diagnosed after 5 years in some
series, emphasizing the need to consider longer follow-
up than advocated in most current surveillance
protocols.
1
Bone metastasis are only rarely identified
during surveillance in the absence of bone pain, an
elevated alkaline phosphatase, or radiographic findings
suggesting a bony neoplasm, and bone scan can
generally be reserved for these indications.
96-99
Patients
with acute neurological signs/symptoms should undergo
prompt cross-sectional imaging of the brain and/or
spine,
370,371,372
but beyond this there is no role for
routine neurologic imaging in surveillance of patients
with localized renal cancer. Additional site-specific
imaging should be ordered as warranted by clinical
signs/symptoms suggestive of recurrence or metastatic
spread. Current data do not support the use of PET
scan in the routine surveillance of patients with renal
cancer, and this test should only be considered
selectively, such as for trouble-shooting when other
tests are concerning but inconclusive.
373
There are no prospective data to compare currently
available surveillance strategies, resulting in substantial
variability in the approach, modality, frequency, and
duration of follow-up after intervention. The premise of
early detection of tumor recurrence after primary
intervention is that this approach will result in patient
cure, improved survival, or appropriate palliation. In
addition, surveillance allows the urologist to provide a
measure of reassurance to the patient who is worried
about cancer recurrence. Surveillance also offers the
opportunity to monitor treatment effects and address
survivorship issues that might arise. Taking all of these
considerations into account, the Panel updated the
follow-up strategies after intervention to strike a useful
and measured balance.
GUIDELINE STATEMENTS
INITIAL EVALUATION AND DIAGNOSIS
Evaluation
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

15
1. In patients with a solid or complex cystic
renal mass, clinicians should obtain high
quality, multiphase, cross-sectional abdominal
imaging to optimally characterize and
clinically stage the renal mass.
Characterization of the renal mass should
include assessment of tumor complexity,
degree of contrast enhancement (where
applicable), and presence or absence of fat.
(Clinical Principle)
Multiphase cross-sectional imaging to assess
enhancement characteristics and the biological potential
of a renal mass should be obtained. The added value of
cross-sectional imaging is to assess for regional tumor
involvement or abdominal metastases, and to exclude
benign AML, which may be distinguished by the
presence of intra-lesional fat.
100
This may be done by
CT or MRI.
101
In rare instances RCC may demonstrate
macroscopic or microscopic fat density on imaging and
even pathologically, but this is the exception rather
than the rule.
102
The risks and benefits of the diagnostic
study should be considered, including risks of radiation
exposure (CT) and contrast administration including
contrast-induced nephropathy or allergic reactions.
Patients with eGFR <45 mL/min/1.73m
2
undergoing CT
with intravenous contrast should be considered for peri-
procedural hydration. Administration of intravenous
contrast should be avoided if possible in patients with
severe CKD who are nearing dialysis. Administration of
intravenous contrast can be used judiciously in patients
on hemodialysis and timed just prior to receiving
dialysis in coordination with nephrology. MRI is
appropriate for patients with contraindications to
iodinated contrast and may provide improved
characterization of small renal tumors, particularly
those less than 2 cm in diameter.
103
The risks of
gadolinium based contrast agents (GBCA) in patients
with altered renal function have been of great interest
since the description of Nephrogenic Systemic Fibrosis,
a potentially lethal fibrosing dermopathy associated
with soft tissue deposition and accumulation of
gadolinium. The risk appears related to the isoform of
gadolinium used with group I GBCA agents having the
highest risk while group II GBCA are associated with
few if any unfounded cases of NSF. A recent systematic
review focused on patients with CKD 4 and 5
reported that the incidence of NSF in this
population was less than 0.07% with second
generation gadolinium agents. Current ACR
guidelines on the use of contrast media state that
patients need not be screened for renal function
prior to receiving group II GBCA, which are now
considered safe at any level of eGFR.
47
Criteria for suspicion of RCC are enhancement of
greater than 15-20 Hounsfield Units on CT or > 20% on
MRI. Adjunctive techniques on MRI can also be utilized
to assess relative risk of malignancy.
101,103
Complex cystic renal masses that have somewhat
thickened irregular walls or septa with measurable
enhancement are classified as Bosniak 3, and
approximately 50% of such lesions are malignant.
Bosniak 4 complex cystic lesions have enhancing
nodular soft tissue components and about 75-90% are
malignant.
104
The recognition that cystic renal masses,
when compared with solid masses, are more likely to
be benign and when malignant less aggressive, has led
to a recent proposed update to the Bosniak
Classification. The update is intended to reduce
interreader variability, improve the precision of
reported malignancy rates, and incorporate MRI into
the classification system.
105
Doppler US and contrast-enhanced US using
microbubbles may also be considered in select patients
in whom other forms of intravenous contrast are
contraindicated. As of 2017, contrast-enhanced US is
approved for assessment of hepatic lesions and can be
considered for off-label use for renal mass
evaluation.
106,107
Imaging should comment on renal mass diameter in
cranio-caudal, transverse, and anterio-posterior
dimensions, tumor morphology, involvement of or
juxtaposition to the renal hilum, vein, or collecting
system, and associated features such as retroperitoneal
lymphadenopathy and presence or absence of
abdominal metastases.
108
Infiltrative growth pattern
can broaden the differential diagnosis and has
prognostic significance. While emerging data suggest
that clear cell RCC may be distinguished from the
papillary subtype by differences in enhancement
patterns, no definitive conclusion can be drawn
regarding biological potential based on enhancement
pattern alone. In addition, significant overlap can exist
in imaging characteristics of RCC and oncocytoma on
cross sectional imaging, or between subtypes of
papillary RCC.
108,109
Several algorithms which quantify aspects of renal
tumor morphometry have been developed to describe
tumor complexity including the relationship with the
renal hilum, collecting system, polarity, and endophytic
versus exophytic location. These systems include the
RENAL nephrometry score, the PADUA score, and the C
-index.
110-112
A number of studies suggest that such
categorization may be useful for selection of type of
surgery (RN or PN) or surgical approach (open or
minimally invasive) as well as provide an estimate of
the risk of surgical complications.
113-115
While some
reports suggest that increasing tumor complexity can
also correlate with aggressive histology or renal
functional outcomes following surgery, the utility of
these systems should be regarded primarily as an aide
for surgical selection and risk stratification for
postoperative complications.
116,117
2. In patients with suspected renal malignancy,
clinicians should obtain a comprehensive
metabolic panel, complete blood count, and
urinalysis. Metastatic evaluation should
include chest imaging to evaluate for possible
thoracic metastases. (Clinical Principle)
Laboratory and metastatic evaluations are important
aspects of the evaluation of the patient with a renal
mass suspicious for RCC. Urinalysis with dipstick and
microscopic evaluation should be obtained to assess for
proteinuria, hematuria, pyuria or signs of other
genitourinary maladies. Presence of proteinuria is an
important prognostic indicator and can be detected by
standard urine dipstick. Patients with a positive dipstick
test (1+ or greater) should undergo confirmation by a
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

16
quantitative measurement (protein-to-creatinine ratio
or albumin-to-creatinine ratio), as part of a focused
medical workup for renal dysfunction.
118,119
The serum
creatinine level should be utilized to calculate an eGFR
by the Modification of Diet in Renal Disease or CKD-EPI
equations.
120,121
Please refer to subsequent statements
regarding patient counseling about functional status,
CKD classification, and management implications
(Guideline Statements 3, 7, 8, 14-17, and 19).
Microscopic hematuria, defined as greater than 3 RBC/
hpf, should also be further assessed to rule out a co-
existing urinary tract conditions.
122
The comprehensive
metabolic panel should be reviewed for electrolyte
abnormalities and hepatic functional parameters.
Abnormalities in hepatic synthetic function may prompt
further workup to exclude co-existing hepatic disease or
metastases which may impact surgical management or
overall prognosis.
123
The presence of elevated alkaline
phosphatase and/or bone pain should spur investigation
of potential bone metastases.
51
Complete blood count
should be considered prior to any intervention.
Initial evaluation of a patient with a renal mass
suspected of malignancy should also include chest
imaging, whether by CT or plain radiography. This is
based on the tumor biology of RCC, with the most
common site of metastatic disease being the chest.
124
While chest CT is more sensitive than plain
radiography, many nonspecific findings (post-
inflammatory or infectious) can also be detected.
Hence, chest imaging should be tailored to tumor risk
with chest radiography being adequate for lower risk
tumors and chest CT being more appropriate in the
setting of higher risk primary tumors (presence of
thrombi, presumed adenopathy, larger tumor size,
infiltrative appearance, or extensive tumor necrosis) or
for patients with relevant symptoms or physical
examination findings.
50,125
3. For patients with a solid or Bosniak 3/4
complex cystic renal mass, clinicians should
assign chronic kidney disease (CKD) stage
based on glomerular filtration rate (GFR) and
degree of proteinuria. (Expert Opinion)
CKD is highly prevalent (approximately 25-30%)
among patients with small renal masses. This
population shares common CKD risk factors including
older age, diabetes mellitus and hypertension.
126-133
All-
cause and cardiovascular mortality increases with CKD
in the general population according to severity of CKD
and even with presence of albuminuria alone.
134,135
Similar association of decreased GFR and/or
albuminuria with increased mortality has been observed
among patients with renal masses (clinical stage T1-
T3).
136
Therefore, identification and proper classification
of CKD as outlined in the Kidney Disease: Improving
Global Outcomes (KDIGO) Guidelines should be
performed. This takes into account: 1) GFR (CKD-EPI
GFR equation); 2) proteinuria; and 3) etiology of
CKD.
121
KDIGO is an independent international non-profit
organization which develops and implements kidney
disease guidelines. First established in 2003, guidelines
regarding CKD classification and management were last
updated in 2012. CKD is diagnosed when renal
functional pathology has persisted greater than 3
months as determined by structural or functional
abnormalities. Beyond identification of CKD, staging
allows for determination of prognosis and stage-related
CKD complications such as hypertension, anemia,
mineral bone disease, metabolic acidosis and
hypoalbuminemia.
137
Additionally, staging allows for
improved risk stratification, functional counseling and
informed decision making.
CKD staging
137
is as follows: 1) eGFR (mL/min/1.73m
2
)
> 90 = G1; G2, 60-89; G3a, 45-59; G3b, 30-44; G4,
15-29; G5 <15; and 2) Albuminuria (Albumin/
creatinine ratio, mg/g)- A1, <30; A2, 30-300; A3
>300. Note that A1 generally correlates with negative
or trace protein on dipstick. Prognosis of CKD is
illustrated in Figure 1.
Figure 1. KDIGO Classification of CKD Risk.
CKD-EPI creatinine clearance equation
(www.mdrd.com): 141 x min(SCr/k, 1)
a
x max(SCr/k,
1)
-1.209
x 0.993
Age
x [1.018 if female] x [1.159 if black],
where SCr is serum creatinine (in mg/dL), k is 0.7 for
females and 0.9 for males, a is 0.329 for females and
0.411 for males, min is the minimum of SCr/k or 1, and
max is the maximum of SCr/k or 1.
121
Renal nuclear scintigraphy measures proportional flow
and function of each kidney which can help assess the
potential impact of renal resection (PN or RN) on global
functional outcomes. Care should be taken when
interpretting the results of renal nuclear scans as pre-
operative proportional GFR assessment may
underestimate actual post-operative GFR due to
technical aspects of scintigraphy, the presence of a
renal mass, hyperfiltration and compensation of the
remaining kidney.
138,139
Recent studies suggest that
differential parenchymal volume analysis may more
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

17
accurately assess split renal function, similar to what is
done for renal donors.
163,272
Counseling
4. In patients with a solid or Bosniak 3/4
complex cystic renal mass, a urologist should
lead the counseling process and should
consider all management strategies. A
multidisciplinary team should be included
when necessary. (Expert Opinion)
Patients diagnosed with a localized renal mass should
have a urologist involved with their care in a leadership
role to help coordinate evaluation, counseling, and
management. Occasionally a multidisciplinary team is
required to further assess and manage the renal mass
based on specific factors.
Patients electing for RMB or percutaneous ablation may
be referred to an interventional radiologist.
Involvement of the urologist in the percutaneous
ablation or RMB procedure appears to depend on local
practice patterns. A survey of 124 academic institutions
in the United States revealed that urologists were
present at the time of percutaneous ablation alongside
the radiologist in 59% of the institutions surveyed.
140
The potential feasibility and safety of office-based US
guided RMB by the urologist has been reported;
however, the vast majority of RMBs are performed by a
radiologist.
141
Given the significant prevalance of CKD in patients with
renal masses that can be exacerbated by surgery or
other treatments, involvement of a nephrologist should
be selectively coordinated. In particular, referral to
nephrology should be considered for patients with eGFR
less than 45 mL/min/1.73m
2
, confirmed proteinuria,
diabetics with preexisting CKD, or whenever eGFR is
expected to be less than 30 mL/min/1.73m
2
after
intervention.
Utilization of RMB in an increasing number of patients
underscores the important role the pathologist plays to
establish an accurate diagnosis. For example, a biopsy
revealing an oncocytic neoplasm may prove to be
benign oncocytoma or an eosinophilic variant of one of
the many subtypes of RCC. Emerging work has
suggested that tissue based molecular markers may aid
in diagnosis or in assigning oncologic risk to a given
tumor.
142,143
Evaluation of the normal adjacent renal
parenchyma for nephrologic disorders can also greatly
enhance patient care. A dedicated pathologist, ideally
with GU subspecialty interest, can be of great value in
the evaluation and management of patients with
localized renal masses.
144-146
A medical oncologist can also be essential for the
management of some patients who present with
clinically localized renal masses, particularly when there
are considerations for neoadjuvant or adjuvant clinical
trials. If final pathology shows high risk or locally
advanced features, adjuvant therapy or clinical trials
should be considered. Additionally, at recurrence these
patients may require systemic therapy. The activity of
neoadjuvant systemic therapies to downsize localized
tumors has been documented in limited clinical
trials.
147,148
Such a strategy may prove helpful for
occasional patients where a nephron-sparing approach
is precluded due to unfavorable tumor size and location
and RN would leave the patient dialysis-dependent.
However, the overall utility of such an approach is
currently unknown.
It is estimated that 4-6% of patients with RCC have a
familial syndrome, and all patients with a renal mass 46
years of age or younger should be referred for genetic
counseling. Patients with multifocal and/or bilateral
renal masses and those with a personal or family
history of malignant or benign findings potentially
associated with the various familial RCC syndromes
should also be strongly considered for genetic
counseling regardless of age. Statement 9 provides
further details regarding specific recommendations for
genetic counseling.
5. Clinicians should provide counseling that
includes current perspectives about tumor
biology and a patient-specific risk assessment
inclusive of sex, tumor size/complexity,
histology (when obtained), and imaging
characteristics. For cT1a tumors, the low
oncologic risk of many small renal masses
should be reviewed. (Clinical Principle)
The current paradigms for patients with clinically
localized renal masses suspicious for malignancy cannot
reliably predict the presence of malignancy or
aggressive tumor biology prior to extirpative surgery.
This includes clinical predictors of malignancy,
adjunctive laboratory tests and RMB. The 2017 AHRQ
report and recent update systematically reviewed the
literature regarding clinical predictors of malignancy
and determined: (1) no composite model of clinical
parameters reliably predicts malignancy, (2) no single
predictive variable (i.e. age, sex) was uniformly
predictive of malignancy; and (3) male sex and
increasing tumor size indicate a higher likelihood of
malignancy.
65
In meta-analysis, male sex imparted a
nearly 3-fold increased risk of malignancy (effect size
2.71, 95% confidence interval 2.39-3.02) compared to
female sex. While benign histology is more common in
women, RCC still predominates in both genders. Across
several studies, tumor size imparted a 30% increased
risk of malignancy per centimeter increase in tumor
size (effect size 1.3 per cm increase in diameter, 95%
confidence interval 1.22-1.43).
65
These findings are
consistent with a wealth of retrospective literature
examining univariate predictors of benign and
malignant pathology in extirpative surgical series.
149
For
example Frank, et al. demonstrated that 46% of tumors
< 1cm are benign and only 2% are high-grade RCC in
contrast to 6% benign and 58% high-grade RCC for
tumors greater than 7 cm.
150
A systematic review by
Johnson et al. demonstrated a decreasing rate of
benign tumors with increasing tumor size from 40% at
1 cm to only 6% for tumors greater than 7 cm.
10
Importantly, many clinical T1a cancers (< 4
centimeters) demonstrate indolent tumor biology. In
retrospective extirpative surgical series, no patient with
a tumor less than 2 centimeters, and less than 2% of
patients with tumors 4 centimeters or smaller,
presented with or developed metastatic disease when
observed for a median of approximately 36 months.
8,125
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

18
The indolent nature of many small and very-small renal
masses (less than 2 cm) is also supported by
prospective AS data, in which 1% or less of patients
progress to metastatic disease.
78,80
Although less robust evidence exists, data also suggest
that tumor architecture, complexity, and enhancement
patterns on imaging may predict malignancy. In the
AHRQ systematic review, solid tumor architecture
(versus cystic architecture) was associated with
malignancy.
65
Increasing tumor complexity (as reported
by the RENAL Nephrometry Score or similar
methodology) was also consistently associated with an
increasing risk of malignancy and aggressive tumor
biology; however, the heterogeneity of these data
prevents meaningful conclusions.
65
A number of studies
indicate that enhancement patterns are predictive of
tumor histology. While papillary RCC is often hypo-
enhancing, both malignant and benign masses can
display heterogeneous avid contrast enhancement
patterns.
109,151
More recent studies have demonstrated
that complex cystic masses, particularly Bosniak 3
category lesions and those that are predominantly
cystic, often have indolent tumor biology and favorable
outcomes on AS.
152-154
In summary, while no model of clinical parameters,
laboratory or radiographic test or RMB reliably predicts
malignancy or aggressive tumor biology, a number of
important pre-treatment parameters can be used to
advise patients about their risk of malignancy and
death from RCC.
65
Consultation should therefore include
a discussion of the influence of patient, imaging, and
tumor characteristics that may impact clinical decision
making. The indolent nature of many small, clinically
localized renal masses should also be reviewed when
relevant.
6. During counseling of patients with a solid or
Bosniak 3/4 complex cystic renal mass,
clinicians must review the most common and
serious urologic and non-urologic morbidities
of each treatment pathway and the
importance of patient age, comorbidities/
frailty, and life expectancy. (Clinical Principle)
The AHRQ report systematically reviewed over 100
manuscripts reporting on the efficacy, comparative
efficacy, and potential morbidities of the four major
management strategies (RN, PN, TA, and AS) for
clinically localized renal masses.
65
The analysis
determined that oncological outcomes are determined
primarily by tumor stage and are similar across
treatment options with the exception of TA. TA was
associated with inferior local recurrence free (LRFS)
survival for primary treatment but equivalent LRFS
following secondary treatments. There was no
significant difference in stage-specific outcomes for well
-selected patients undergoing any of the management
strategies, with the important caveat that the majority
of patients undergoing TA or AS had small renal masses
with less biological aggressiveness. A key finding in
reviewing these data is that overall survival is
determined primarily by age and risk of competing
comorbidities.
65
A number of retrospective analyses
confirm these findings, indicating that competing risk
mortality exceeds cancer-specific mortality for many
patients with clinically localized tumors, and that this is
largely driven by cardiovascular comorbidities.
-,,
Therefore, cancer-specific survival is primarily
determined by tumor characteristics and overall
survival is determined by patient age and competing
risk of comorbidities, specifically cardiovascular
comorbidity in the population with clinically localized
renal cancer.
65
Each management strategy for the solid or complex
cystic mass is associated with a unique profile of renal
functional outcomes, perioperative outcomes, potential
harms, and health-related quality of life. It should be
noted that each treatment strategy (RN, PN, or TA) has
similar rates of minor and major complications but a
unique profile of these complications that should be
discussed with patients.
65
Selection of a management
strategy should therefore take into account patient
preferences and prioritize potential harms associated
with each management strategy on an individual basis.
RN is associated w ith the greatest decrease
in GFR and highest risk of de novo CKD stage 3 or
higher. While these changes in GFR may be
clinically insignificant in patients with a normal
contralateral kidney, they warrant consideration
and discussion in certain patients. RN is associated
with favorable perioperative outcomes and a low
risk of urologic complications compared to PN.
65
The
favorable outcomes associated with RN may reflect
the high proportion of RN performed via the
minimally invasive approach.
PN offers excellent preservation of renal
parenchyma and GFR; however, it carries a higher
risk of blood transfusions and urologic complications
(e.g., urine leak) than other modalities. These
complications may subject a small proportion of
patients to additional treatments (e.g., ureteral
stents, abdominal drains, embolization of
pseudoaneurysm).
65
TA carries an inferior LRFS w hen considering
primary efficacy that may mandate secondary
interventions. In the AHRQ analysis,
65
TA had the
most favorable perioperative outcome profile and a
similar low risk of harms when compared to other
strategies. Success rates with TA are highest with
small peripheral tumors.
AS offers favorable oncologic and overall
survival outcomes in well-selected patients, albeit
in limited studies with relatively short to
intermediate-term follow-up.
78,80,159-161
AS foregoes
the operative risks associated with other
management strategies but potentially introduces
anxieties and oncologic risks not suitable for all
patients.
The AHRQ analysis and literature update was unable to
identify strong, consistent predictors of comparative
benefit among management strategies due to
heterogeneity and paucity of data, particularly in
treatments other than RN or PN.
65
Increasing age or
limited life expectancy is associated with lower
incidence of cancer-specific mortality independent of
management strategy. This phenomenon is most robust
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

19
in patients greater than 75 years of age, where the
comparative benefits of intervention and subsequent
detriments of decreases in GFR are more difficult to
quantify. Therefore, it is impossible to make a blanket
statement that one management strategy is preferred
based on patient age, comorbidities, frailty, and/or life
expectancy, but all should be considered during
individualized counseling.
65
7. Clinicians should review the importance of
renal functional recovery related to renal
mass management, including the risks of
progressive CKD, potential short- or long-term
need for renal replacement therapy, and long-
term overall survival considerations. (Clinical
Principle)
Individuals with localized renal masses have a high
burden of CKD to begin with, partially because this
population shares risk factors that are common to CKD.
They tend to be older with high prevalence of diabetes
mellitus (10-20%) and hypertension (25-50%). Poorly
controlled diabetes mellitus and hypertension can
induce hyperfiltration and glomerular hypertension
resulting in CKD or exacerbation of CKD leading to
further loss of function. After surgical resection, CKD
prevalence and degree further increases.
126-129,162
Most
studies suggest that patients with CKD due to medical
etiologies have reduced overall survival and are at
increased risk for cardiovascular events. Patients with a
renal mass and preexisting CKD are at increased risk
for progressive decline in renal function after surgery
and also may experience increased mortality rates.
However, recent studies suggest that patients with CKD
that is primarily due to surgical removal of nephrons,
rather than medical causes, may have better outcomes,
as long as the new baseline GFR is greater than 45 mL/
min/1.73m
2
.
163,164
Almost all studies in this domain are
retrospective and further investigation is required.
165
8. Clinicians should consider referral to
nephrology in patients with a high risk of CKD
progression, including those with estimated
glomerular filtration rate (eGFR) less than 45
mL/min/1.73m
2
, confirmed proteinuria,
diabetics with preexisting CKD, or whenever
eGFR is expected to be less than 30 mL/
min/1.73m
2
after intervention. (Expert
Opinion)
Predictive factors for post-operative development of
CKD or progression of pre-existing CKD include older
age, diabetes mellitus (DM), hypertension (HTN), as
well as male sex, obesity, tobacco use, larger tumor
size, and post-operative acute kidney injury.
128,133,166-170
Patients who present with eGFR less than 45 mL/
min/1.73m
2
or confirmed proteinuria are at particularly
high risk from a functional standpoint, and should be
considered for nephrology consultation. Patients who
are expected to have an eGFR less than 30 mL/
min/1.73m
2
after intervention will also be at high risk
long-term, and a nephrologist should be involved in
their care. Identifying modifiable risk factors including
DM, HTN and smoking is essential. Optimizing glycemic
and blood pressure control, smoking cessation and
minimizing risk of acute kidney injury (with avoidance
of hypotension and nephrotoxic agents such as
intravenous contrast or non-steroidal anti-inflammatory
drugs) should reduce the degree of renal dysfunction in
the perioperative period.
171
Of note, patients with DM
are at even higher risk for AKI compared with those
without DM, even among those with normal eGFR prior
to nephrectomy.
128
With significant nephron mass loss, hyperfiltration can
occur resulting in glomerular damage, exacerbation of
proteinuria and progressive sclerosis with further
decline in GFR.
,
Therefore, repeat assessment of blood
pressure, eGFR, and proteinuria should be performed
soon after nephrectomy then again in 3-6 months to
assess for development or progression of CKD. With
any compromise in eGFR or presence of CKD
complications, additional regular monitoring of kidney
function should be performed and further management
of CKD would be recommended with referral to
nephrology. Careful management of DM and HTN and
avoidance of substantial weight gain may slow or
prevent CKD progression and should be prioritized on a
long-term basis.
120,121,144,145,174-176
9. Clinicians should recommend genetic
counseling for any of the following: all
patients ≤ 46 years of age with renal
malignancy, those with multifocal or bilateral
renal masses, or whenever 1) the personal or
family history suggests a familial renal
neoplastic syndrome; 2) there is a first-or
second-degree relative with a history of renal
malignancy or a known clinical or genetic
diagnosis of a familial renal neoplastic
syndrome (even if kidney cancer has not been
observed); or 3) the patient’s pathology
demonstrates histologic findings suggestive of
such a syndrome. (Expert Opinion)
Recognition of familial forms of RCC can be of great
benefit to patients and their families. Genetic
counseling is typically pursued after biopsy or surgery
has been performed and pathology is available to guide
future testing. If positive, other manifestations of the
various syndromes can be identified and family
members can also be considered for genetic testing.
19
Proactive management of RCC and other familial
manifestations may considerably lessen the morbidity
and mortality associated with these syndromes.
19
Improved understanding of specific hereditary forms of
RCC has resulted in well-defined recommendations
regarding the role of AS, appropriateness of nephron-
sparing surgery, and timing of intervention for the
various syndromes.
19,177
For example, patients with VHL
rarely experience a metastasis when their tumors are
less than 3 cm, and are thus typically observed until
the largest tumor crosses this size threshold.
39
This is in
contrast to patients with HLRCC who usually present
with aggressive cancers that should trigger prompt
aggressive intervention.
178
While it is estimated that 4-6% of patients with RCC
may have a familial syndrome, some studies suggest
that contributing genetic mutations may be even more
common than previously appreciated, and referral for
genetic counseling should be considered more often
than in the past.
179
A positive family history (first-or
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

20
second-degree relative with a history of renal
malignancy or a known clinical or genetic diagnosis of a
familial renal neoplastic syndrome, even if kidney
cancer has not been observed) should prompt referal
for genetic counseling. Identification of classic
manifestations of known familial syndromes is also a
strong indication for genetic evaluation.
180-182
Several
RCC syndromes have been characterized and are listed
in Table 5 along with their clinical correlates:
Patients presenting with bilateral or multifocal RCC
should also be considered for genetic counseling, as
should those with uncommon but characteristic tumor
histologies such as hybrid oncocytic/chromophobe
tumors suggestive of BHD. Other pathologic findings
that should prompt consideration for genetic counseling
include histology suggesting HLRCC/fumarate hydratase
deficiency or SDH deficient RCC, or AML in the presence
of one or more additional TS complex criteria.
180-182
Since sporadic RCC typically presents at a more
advanced age than hereditary RCC, patients presenting
at a young age should also be considered for genetic
evaluation. One important study of the SEER cohort
revealed that the median age of onset of sporadic RCC
was 64 years compared to 37 for those with hereditary
disease.
182
Based on this data, it was recommended
that patients diagnosed at the age of 46 years or
younger should be strongly considered for genetic
counseling.
Renal Mass Biopsy (RMB)
10. When considering the utility of RMB, patients
should be counseled regarding rationale,
positive and negative predictive values,
potential risks and non-diagnostic rates of
RMB. (Moderate Recommendation; Evidence
Level: Grade C)
RMB is an important diagnostic adjunct for selected
patients with renal masses suspicious for clinically
localized renal cancer. Patients seeking additional
information regarding their diagnosis or clinicians
needing more information may elect RMB for histologic
data to enhance counseling and clinical decision
making. Before undergoing RMB, consultation regarding
the performance characteristics and risks of RMB should
be undertaken. First, patients should understand that
RMB is generally a safe diagnostic test. The risk of
complications is low with the most common being renal
hematoma (4.9%), clinically significant pain (1.2%),
gross hematuria (1.0%), pneumothorax (0.6%) and
hemorrhage requiring transfusion (0.4%).
183-190,200
While the risk of post-procedure hemorrhage is small,
these risks may be amplified by aspirin, NSAIA, second
or third generation antiplatelet agents (i.e.
dipyridamole, clopidogrel), vitamin K/factor X inhibitors
(i.e. warfarin, apixaban), and low molecular weight
heparin (i.e. enoxaprin). Temporary discontinuation of
these agents is advised if the risk/benefit ratio allows.
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Table 5. Familial RCC Syndromes.
*Renal cancers associated with these syndromes are typically or often more aggressive
Syndrome Gene Clinical Manifestations
Von Hippel-Lindau (VHL) VHL Clear cell RCC, renal cysts, hemangioblastomas of the central
nervous system, retinal angiomas, pheochromocytoma
Hereditary Papillary Renal Car-
cinoma (HPRC)
MET Type 1 papillary RCC
Birt Hogg-Dubé (BHD) FLCN Chromphobe RCC, oncocytoma, hybrid oncocytic/
chromophobe tumors (HOCTs), clear cell RCC (less common),
renal cysts, cutaneous fibrofolliculomas, lung cysts, spontane-
ous pneumothorax
Hereditary Leiomyomatosis
and RCC (HLRCC)*
FH Type 2 papillary or collecting duct RCC, cutaneous leioyomyo-
mas, uterine leiyomyomas
Succinate Dehydrogenase Kid-
ney Cancer (SDH-RCC)*
SDHB/C/D Clear cell RCC, chromophobe RCC, type 2 papillary RCC, on-
cocytoma , pheochromocytoma/paraganglioma
BAP-1 Tumor Predisposition
Syndrome*
BAP-1 Clear cell RCC, uveal melanoma
Tuberous Sclerosis Complex
(TSC)
TSC1/2 AML, clear cell RCC, oncocytoma, lymphangioleiyomyomasto-
sis (LAM), seizures, developmental delay
Cowden/PTEN Syndrome Asso-
ciated RCC (CS-RCC)
PTEN Thyroid, breast, and endometrial cancers, mucocutaneous
lesions, RCC with papillary most common, also other forms of
RCC, including clear cell

21
Importantly, there are no reported cases of RCC tumor
seeding in the contemporary literature with modern
biopsy techniques, which typically utilize a coaxial
sheath. In addition, patients should be informed that a
RMB diagnostic of malignancy and histologic subtype
tends to be highly accurate.
Based on a bivariate meta-analysis of seven
studies that compared diagnosis using RMB with
surgical pathology, the sensitivity (96.7% (95%
CI 93.8-98.2%)), specificity (94.4% (95% CI
71.9–99.1%)), and positive predictive value
(98.8% (95% CI 97.0–99.5)) of core RMB are
excellent and a diagnosis of malignancy can be
trusted with certainty (Figure 2). I n addition,
histologic determination of RCC subtype is highly
accurate.
200,201
However, patients should be informed
that the non-diagnostic rate of RMB is approximately
14%, which can be substantially reduced with repeat
biopsy.
185-191,201
Another concern with RMB has been
histologic heterogeneity, particularly for benign tumors
such as oncocytomas. In these cases there may be a
concurrent focus of cancer (i.e. hybrid oncocytic tumors
with chromophobe RCC), which could lead to misleading
RMB results.
192
However, recent studies suggest that
this does not substantially alter the outcomes for most
such patients.
Pooled estimates of sensitivity and specificity and their
95% confidence intervals were modelled using the
metandi module in StataMP v14. Metandi performs
bivariate meta-analyses of sensitivity and specificity
using a hierarchical modeling approach.
On the other hand, RMB carries a concerning negative
predictive value (NPV 80.8%, 95% CI 70.1-88.3%),
suggesting that a non-malignant biopsy result may not
truly indicate that a benign entity is present. In the
systematic review performed by Patel et al., the NPV
was 63% indicating that among patients undergoing
extirpation despite a negative biopsy, 37% had
malignant disease on final surgical pathology.
200
As this
comprised a select population with high risk clinical and
imaging features, it likely represents the upper limit of
NPV for RMB. In addition, the accuracy of tumor grade
diagnosis with RMB is highly variable, ranging from 52-
76% in the literature. Sixteen percent (16%) of tumors
were upgraded from low-grade to high-grade at
surgical pathology. This is particularly pertinent for
patients with small renal masses, where 80-90% of
tumors are low-grade and the detection of high-grade
tumors is of paramount importance. Hence, this
represents a significant limitation of RMB.
185-190
Furthermore, oncocytic neoplasms may present a
challenge for RMB (i.e., differentiating chromophobe
RCC vs. oncocytoma). A summary of recommended
issues for emphasis during counseling about RMB is
listed below.
192,193
DISCUSSION POINTS FOR RMB:
RMB is generally safe with low risk of significant
complications (bleeding) and no reported cases of
tumor seeding using contemporary techniques.
A diagnosis of malignancy or RCC on RMB is highly
reliable.
Potential limitations of RMB include:
A benign biopsy must be distinguished from a non-
diagnostic biopsy (renal parenchyma or connective
tissues) result.
A benign biopsy may not always correlate with
benign histology.
Grade concordance from biopsy to surgically
resected tissue is imperfect.
Oncocytic neoplasms may represent a diagnostic
dilemma.
Biopsy or aspiration of cystic renal masses is
generally not advised due to concerns regarding
tumor spillage and a high likelihood of obtaining a
non-informative result due to sampling error.
11. Clinicians should consider RMB when a mass is
suspected to be hematologic, metastatic,
inflammatory, or infectious. (Clinical
Principle)
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Figure 2. Reported sensitivity and specificity for a malignant diagnosis using core RMB when com-
pared with surgical pathology.
Pooled sensitivity: 96.67%; 95%CI, 93.79-98.24%; Pooled specificity: 94.45%; 95%CI, 71.93-99.13%

22
Patients presenting with an enhancing renal mass
should be considered for RMB if: 1) there is suspicion
that the lesion represents metastatic cancer from
another primary source; 2) the radiographic or clinical
picture suggests hematologic malignancy involving the
kidney; or 3) there is concern for an inflammatory or
infectious process. Although metastatic cancer involving
the kidney is frequently found at autopsy, clinical
presentation of renal metastases is uncommon. The
most common hematologic malignancy to involve the
kidney is lymphoma and the most common solid tumor
metastasis is lung cancer, although melanoma, colon
cancer and thyroid cancer have also been reported.
194
In patients with a prior history of malignancy with
potential renal metastasis, or in those with an atypical
renal mass and concerning constitutional symptoms,
RMB should be considered.
195
If metastatic cancer is
confirmed, systemic treatment is typically prioritized.
194
Metastases to the kidney are often multifocal, poorly
enhancing, and infiltrative rather than well demarcated,
although there are exceptions to these rules. Renal
lymphoma should be considered in patients with
infiltrative renal lesions, in those with lymphadenopathy
that is out of proportion to the renal primary, or when
the anatomic distribution of involved nodes is markedly
atypical for RCC. In contrast, patients with a solitary,
avidly enhancing renal mass and a remote history of
cancer will likely have RCC and can be managed
accordingly.
19
In patients presenting with signs and symptoms
consistent with an infectious or inflammatory condition
or those with a prior history of recurrent infections or
autoimmune disease, the clinician’s index of suspicion
for a non-neoplastic process, such as renal sarcoidosis,
abscess, or focal pyelonephritis, should be increased. In
this setting, RMB should be considered for diagnostic
purposes and to direct therapy.
196-199
12. In the setting of a solid renal mass, RMB
should be obtained on a utility-based
approach whenever it may influence
management. RMB is not required for 1)
young or healthy patients who are unwilling to
accept the uncertainties associated with RMB;
or 2) older or frail patients who will be
managed conservatively independent of RMB
findings. (Expert Opinion)
Patients with a renal mass should be counseled about
the differential diagnosis including the likelihood of
malignant versus benign histology. A utility-based
approach is recommended for RMB, which is not
indicated when it is unlikely to alter management
recommendations or patient choice.
200,201
Patients with
severe CKD often have benign or indolent tumors and
their management can be complex, and this represents
one of the cohorts that should be strongly considered
for RMB.
202
RMB should also be considered in patients
for whom it is difficult to decide between management
with PN versus RN, where additional oncologic risk
stratification may be helpful. Many young or healthy
patients are unwilling to accept the potential
uncertainty of RMB such as the possibility of a non-
diagnostic or false negative result, and will elect
intervention regardless of RMB outcome.
75
Some older
or frail patients are not healthy enough to undergo
intervention and will be managed conservatively even if
RMB suggests malignancy.
75,80,200,201
In these settings,
RMB is typically not required because it will not
materially alter counseling or management. Please refer
to guideline statements 10 and 13, which include
pertinent details regarding the processes, risks and
performance characteristics of RMB and further
considerations for patient counseling.
13. For patients with a solid renal mass who elect
RMB, multiple core biopsies should be
performed and are preferred over fine needle
aspiration (FNA). (Moderate
Recommendation; Evidence Level: Grade C)
RMB may be performed under CT or US guidance, with
at least 2-3 cores being obtained with a 16-18 gauge
needle to optimize diagnostic yield. FNA is associated
with a decreased diagnostic yield and core biopsy is
preferred when feasible. The Patel et al. systematic
review of RMB demonstrated core biopsy to have a
sensitivity of 97.5% while the sensitivity of FNA was
reported at 62.5%.
193
The diagnostic rate of RMB is
dependent upon obtaining viable tissue from the lesion
in question. The American Society of Cytopathology
endorses Rapid On-Site Evaluation (ROSE), which can
optimize specimen quality for pathologic evaluation by
obtaining real-time assessment of FNA or touch
imprints of core biopsies to confirm specimen
adequacy.
203
However, the additional challenges for
workflow and personnel issues to implement ROSE are
also recognized and such techniques are important but
not currently considered mandatory.
MANAGEMENT
Partial Nephrectomy (PN) and Nephron-Sparing
Approaches
14. Clinicians should prioritize PN for the
management of the cT1a renal mass when
intervention is indicated. In this setting, PN
minimizes the risk of CKD or CKD progression
and is associated with favorable oncologic
outcomes, including excellent local control.
(Moderate Recommendation; Evidence Level:
Grade B)
PN is a definitive surgical procedure that is associated
with excellent oncological and renal functional
outcomes, particularly in patients with small renal
masses. It also yields complete pathological information
regarding the excised tumor and minimizes the
oncological uncertainty that can occasionally be
associated with repeat sessions of TA. PN is associated
with urologic complications in a small proportion of
patients but most can be successfully managed with
conservative measures.
204
The EORTC randomized trial suggests that PN is
associated with similar oncological outcomes when
compared to RN for clinically localized small (<5cm)
renal masses, and the AHRQ systematic review and
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

23
update has reaffirmed this for carefully selected
patients.
204,205
Meta-analysis of the existing data further
documents that PN is associated with less decline in
postoperative GFR and a lower incidence of CKD stage
3 or above when compared to RN (figures 3 and 4).
65
PN is also associated with more favorable local
recurrence-free survival when compared to a single
session of TA (Figure 5). While patients undergoing PN
have a higher risk of blood transfusion and urological
complications, the overall complication rates
experienced by patients undergoing PN are similar to
other treatment modalities and can be minimized in
experienced hands. Given uncertainties regarding
future development of CKD, the increasing prevalence
of CKD risk factors (obesity, hypertension, tobacco use)
related to RCC in the general population, the risk of
recurrent or de novo disease in the contralateral renal
unit,
206
and the indolent nature of most small kidney
tumors, PN should be prioritized in the management of
patients with clinical T1a renal mass.
176,207-212
15. Clinicians should prioritize nephron-sparing
approaches for patients with solid or Bosniak
3/4 complex cystic renal masses and an
anatomic or functionally solitary kidney,
bilateral tumors, known familial RCC,
preexisting CKD, or proteinuria. (Moderate
Recommendation; Evidence Level: Grade C)
Absolute indications for PN included situations in which
RN would render the patient anephric or at high risk for
renal replacement therapy. These include patients with
an anatomic or functionally solitary kidney, bilateral
tumors, or known familial RCC. While most patients
with familial RCC have two functional renal units, they
are very likely to experience bilateral tumors, tumor
recurrence and require multiple renal surgeries
throughout their lifetime.
38
The importance of nephron
sparing approaches and thresholds for intervention (i.e.
3 cm) for most RCC syndromes have been well
established through the experience at the National
Cancer Institute.
213
PN in patients with absolute
indications should focus on preservation of renal
parenchymal volume and functional nephrons with
margin width being a less relevant consideration.
214
Approximately 25-30% of a well-functioning, solitary
kidney is generally sufficient to avoid renal replacement
therapy and therefore, overall preservation of renal
function is achievable in most patients with absolute
indications for PN.
215,216
All patients with an absolute
indication for PN should be advised about the potential
need for temporary or permanent renal replacement
therapy following surgery. In one series of solitary
kidneys managed with PN, rates of temporary and
permanent end-stage renal failure were 3.5% and
4.5% respectively.
217
Another study of solitary kidneys
reported acute renal failure in 12.7% of patients, and
proteinuria and significant CKD in 15.9% and 12.7% of
patients, respectively.
218
Traditional relative indications for PN have included
patients with conditions that would threaten future
function of a contralateral renal unit such as preexisting
CKD and proteinuria. In the 2016 AHRQ report of
patients with normal contralateral kidneys, rates of end
-stage renal disease (ESRD) for RN, PN, and TA were 1-
3%, 0.4-1%, and 1-2%, respectively.
65
However, the
current literature suggests that patients with pre-
existing CKD and proteinuria are at highest risk for
progressive CKD and ESRD.
219-221
It is noteworthy that
patients with proteinuria, even without a decrease in
GFR, are at increased risk of progressive loss of renal
function.
222
Therefore, PN should also be prioritized in
these patients.
16. Nephron-sparing approaches should be
considered for patients with solid or Bosniak
3/4 complex cystic renal masses who are
young, have multifocal masses, or
comorbidities that are likely to impact renal
function in the future, including but not
limited to moderate to severe hypertension,
diabetes mellitus, recurrent urolithiasis, or
morbid obesity. (Moderate Recommendation;
Evidence Level: Grade C)
The EORTC 30904 randomized trial of RN versus PN
demonstrated higher eGFR in patients undergoing PN
compared to RN: 66.8 versus 52.7 mL/min/1.73m
2
within the first year, respectively. However, there was
no evidence of subsequent decline in eGFR in either
surgical cohort and the rates of end stage renal disease
(eGFR less than 15 mL/min/1.73m
2
) were 1.5% and
1.6% respectively.
223
However, this was a population of
aged adults (median age >60 years old) in generally
good health with normal contralateral kidneys
(preoperative serum creatinine <1.25 mg/dL in >90%)
and thus should not be extrapolated to all patients with
clinically localized renal masses. Younger patients who
have longer life expectancy are theoretically at risk of
recurrent and/or contralateral disease as well as
competing health risks that can impact renal function
over their extended remaining life time. For this reason,
these patients should undergo nephron-sparing
approaches whenever technically feasible. In
reasonably healthy patients managed by experienced
surgeons, the risks of nephron sparing surgery are low
and balance the uncertainties of recurrent disease or
the development of unforeseen health issues. Patients
with multifocal tumors often have familial RCC and
should be managed as such.
38
They will typically
require multiple renal interventions throughout their
lifetime.
38
For these patients, the importance of
nephron sparing approaches and thresholds for
intervention have been well established through the
experience of the National Cancer Institute.
213
Lastly,
patients with significant risk for future CKD such as
patients with severe hypertension, diabetes mellitus,
strong stone diathesis, or morbid obesity should be
considered for nephron-sparing approaches in order to
optimize their renal function.
224-226
The risks of CKD
should be discussed with patients keeping in mind that
oncologic outcomes should remain a priority.
17. In patients who elect PN, clinicians should
prioritize preservation of renal function by
optimizing nephron mass preservation and
avoiding prolonged warm ischemia. (Expert
Opinion)
One of the main objectives of PN is to preserve renal
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
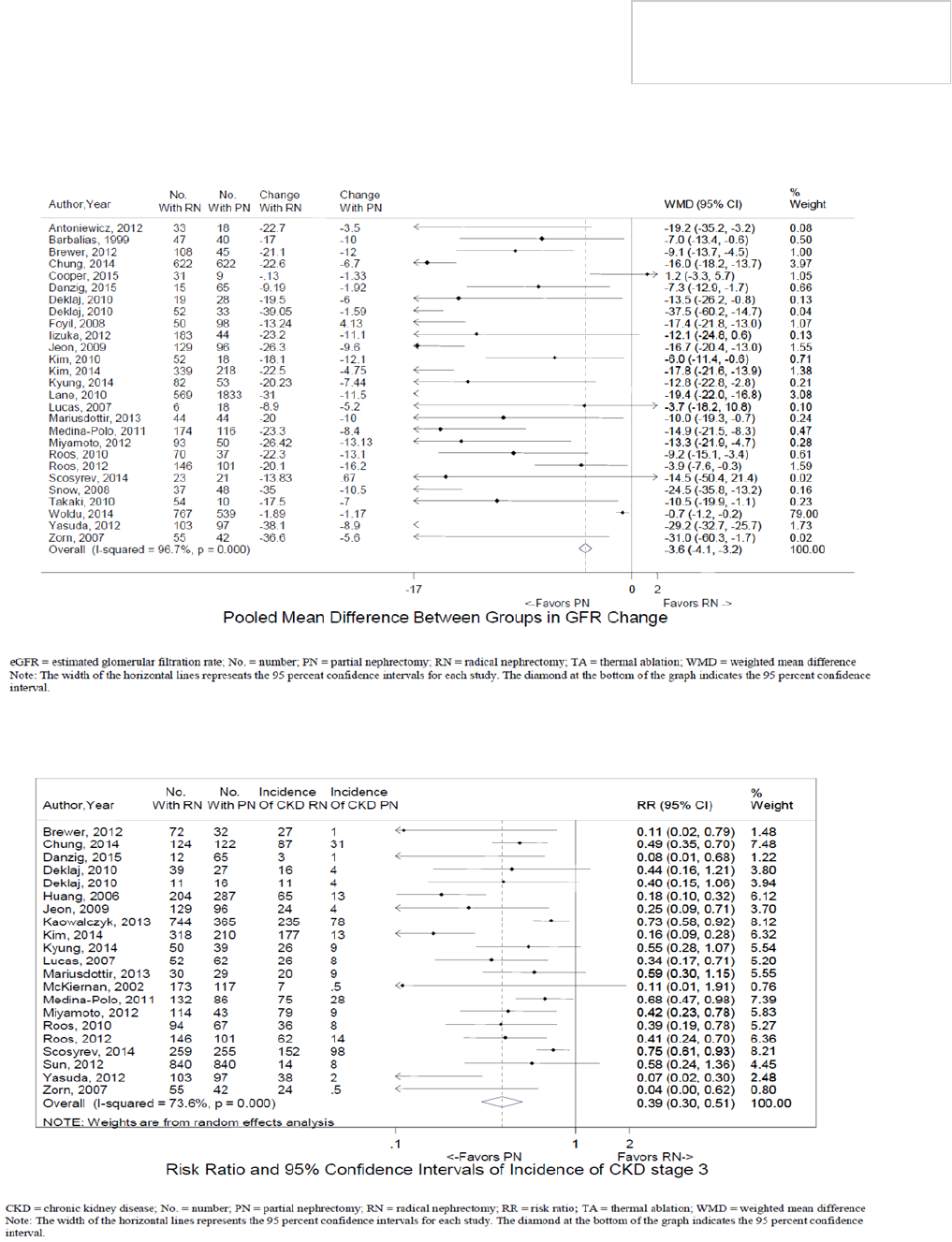
24
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Figure 3. Mean change in eGFR for RN versus PN.
65
Figure 4. Meta-analysis of the incidence of stage 3 CKD with RN versus PN.
65

25
Figure 5. Meta-analysis of local recurrence rates for PN versus primary TA among studies with follow-up
of 48 months ± 12 months.
65
Abbreviations: CI, confidence interval; IV, inverse variance; PN, partial nephrectomy; TA, thermal ablation.
Note: Total patients is defined as all patients with biopsy proven RCC treated with each modality. Events refer
to number of patients with local recurrence.
with a solitary kidney, bilateral or multifocal disease, or
preexisting CKD or proteinuria.
214,227-229
However, even
when PN is performed electively, there may be value in
optimizing renal function on a long-term basis. Current
studies regarding the impact of incremental changes in
renal function related to renal cancer surgery on overall
survival do not extend beyond 10 years follow-
up,
165,219,220
but both the randomized trial of PN versus
RN and a plethora of comparative, retrospective data
indicate worse overall GFR and higher rates of CKD
stage ≥3 in patients undergoing RN.
65,223
In addition,
uncertainties regarding development of CKD in patients
without risk factors and the low, but tangible risk of
developing contralateral masses are reasons to consider
PN and other nephron sparing approaches when
technically feasible and have high likelihood of
success.
206
The recent literature demonstrates that the main
determinant of functional outcomes after PN is nephron
mass preservation, or the quantity of vascularized
parenchyma that is preserved by the procedure.
227-231
Efforts to optimize this parameter during tumor excision
and reconstruction should be prioritized, as long as
oncologic outcomes are not compromised.
232
Beyond this, prolonged warm ischemia should be
avoided, as it can lead to irreversible loss of function.
The exact threshold of warm ischemia at which
irreversible damage begins to occur is not well defined,
although some studies suggest that some patients may
begin to experience this to a significant degree at
approximately 25-30 minutes.
227-229
In general,
recovery from hypothermia is more consistent and
reliable with intervals up to 60-90 minutes being well
tolerated.
233
Nevertheless, even with hypothermia it is
best to avoid truly prolonged durations of ischemia, as
they can lead to increased risk of acute kidney injury,
which may complicate postoperative care.
231,234,235
Avoidance of ischemia or segmental clamping are other
strategies that have been advocated in an effort to
obviate ischemia injury.
227,236-238
Such approaches can
be supported as long as nephron mass preservation
remains strong and perioperative and oncologic
outcomes are not compromised.
239,240
18. For patients undergoing PN, clinicians should
prioritize negative surgical margins. The
extent of normal parenchyma removed should
be determined by surgeon discretion taking
into account the clinical situation and tumor
characteristics, including growth pattern, and
interface with normal tissue. Tumor
enucleation should be considered in patients
with familial RCC, multifocal disease, or
severe CKD to optimize parenchymal mass
preservation. (Expert Opinion)
The primary goal of PN is complete tumor excision and
as such achieving negative surgical margins should
remain a priority. Positive surgical margins introduce
oncological uncertainty and cause patient anxiety.
Recent studies have suggested inferior oncological
outcomes in patients with positive surgical margins
after PN.
241,242
Preservation of renal parenchyma is among the
strongest predictors of functional outcomes after PN
and is thus particularly important in patients with
severe CKD or a propensity for multifocal and bilateral
RCC.
221
The amount of normal tissue excised during PN
should be determined by surgeon judgment taking into
account patient and tumor characteristics. The concept
of tumor enucleation (or blunt excision of a tumor with
minimal margin during nephron-sparing surgery)
originated in the familial RCC population as a technique
to preserve renal parenchyma in patients with multiple
tumors requiring several surgeries over a lifetime.
243
However, even for familial RCC patients tumor
enucleation should be applied selectively. For example,
some syndromes, such as here HLRCC, tend to have
unifocal aggressive tumors and are best managed with
wide margin PN or RN.
Enucleation has subsequently been evaluated in the

26
function, and this is particularly important in patients
sporadic RCC population with a number of studies
reporting similar oncological outcomes compared to
traditional PN, in which sharp excision is performed
with intentional removal of a modest rim of normal
adjacent parenchyma.
244-247
Most studies comparing
enucleation and traditional PN have been retrospective
and uniform pathologic review has not been applied.
Selection for enucleation based on favorable imaging
characteristics such as homogeneity and encapsulated
appearance is likely another contributing factor in many
of these studies.
248
In addition, tumor enucleation is
based on the concept of blunt dissection along a tumor
pseudocapsule, which is present in many but not all
renal cancers.
249-251
When present, the pseudocapsule
can contain invasive cancer in up to one third of cases
with an unclear influence on prognosis.
252
Given these
concerns, great care should be taken to assess tumor
growth pattern and its interface with the normal
parenchyma to assess feasibility for successful
enucleation. Until prospective evaluation is available for
sporadic renal tumors, enucleation is best utilized on a
selective basis.
Frozen section analysis of the margins during PN or
tumor enucleation can be considered on a selective
basis, particularly when there is concern about the
gross specimen. The management of positive surgical
margins after PN or tumor enucleation remains
controversial. A variety of factors should be taken into
account during counseling including the extent of the
margin (microscopic versus extensive), tumor histology
and grade, and other indicators of tumor biology such
as locally invasive phenotype. Most patients with
microscopic positive surgical margins associated with
small renal masses tend to do well with expectant
management, although close surveillance is
recommended.
253
Radical Nephrectomy (RN)
19. Clinicians should consider RN for patients with
a solid or Bosniak 3/4 complex cystic renal
mass whenever increased oncologic potential
is suggested by tumor size, RMB (if obtained),
and/or imaging. (Moderate Recommendation;
Evidence Level: Grade B) In this setting, RN is
preferred if all of the following criteria are
met: 1) high tumor complexity and PN would
be challenging even in experienced hands; 2)
no preexisting CKD or proteinuria; and 3)
normal contralateral kidney and new baseline
eGFR will likely be greater than 45 mL/
min/1.73m2 even if RN is performed. If all of
these criteria are not met, PN should be
considered unless there are overriding
concerns about the safety or oncologic
efficacy of PN. (Expert Opinion)
Many cT1b/T2 tumors can be considered for PN, and
observational studies suggest that acceptable outcomes
can be achieved with PN in this setting, assuming
appropriate patient selection and surgical experience.
254
-261
However, oncologic potential correlates with tumor
size as reflected by increased incidence of high grade
tumor, less favorable histology, and locally advanced
features.
150,262
Infiltrative appearance on imaging also
suggests high grade tumor and/or poorly differentiated
elements, including sarcomatoid features.
263,264
In this
setting PN may place the patient at increased risk of
local recurrence
265
and thus RN may provide an
oncologic advantage.
259,262
Another major consideration for some cT1b/T2 tumors
relates to feasibility of PN, particularly if tumor
complexity is increased related to hilar tumor location.
Urologic complications such as urine extravasation and
postoperative bleeding are more common after PN for
high complexity cases.
266,267
In this setting referral to a
more experienced colleague or center should be
considered to assess feasibility of PN. If PN appears to
be challenging even in experienced hands RN should be
considered, particularly if oncologic indicators are
unfavorable as discussed above.
259,262
The other important consideration for such patients is
functional, and recent studies suggest that there is a
subgroup of patients who experience relatively
favorable outcomes with RN, even if they develop CKD
after surgery.
165,219,220,223
Such patients have no
preexisting CKD (baseline GFR > 60mL/min/1.73m
2
),
no proteinuria (dipstick negative or trace), and a
normal contralateral kidney that is expected to provide
an eGFR of greater than 45 mL/min/1.73m
2
after RN.
Patients with CKD primarily due to surgery who meet
the above criteria appear to have overall survival and
stability of renal function during intermediate-term
follow-up (approximately 10 years) similar to those
without CKD even after surgery.
165,219,220
Results of
EORTC 30904 also supports good survival in select
patients managed with RN even if they develop CKD
after surgery. Overall survival in this study with over a
decade of follow-up was almost identical in the RN and
PN cohorts, even though the median new baseline GFR
in the RN cohort was 52 mL/min/1.73m
2
, confirming
that most RN patients had CKD after surgery.
A related consideration when deciding about the utility
of PN is the amount of parenchymal mass that will be
preserved with the procedure. Some large centrally
located tumors have already replaced a substantial
proportion of the kidney, and in this setting PN may
yield a remnant kidney with only marginal function
after excision and reconstruction have been
accomplished.
259
In general, median loss of global renal
function with PN is about 10%, while RN is typically
associated with about 35-40% median loss of global
function, although this can vary substantially for RN
based on uneven split renal function, and for PN based
on tumor complexity, as discussed above.
228
Patients who combine all of the salient features in
Statement 19 should be considered for RN as these are
patients for whom RN may provide an oncologic benefit
with very little downside, and in whom the oncologic
and perioperative risks of PN would be increased.
Beyond these circumstances, PN is generally preferred
for surgical excision. However, in some patients who do
not meet the composite profile in Statement 19, PN
may not be possible or advisable even in experienced
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

27
hands. In this setting RN may be required based on
surgeon discretion, with input from other services such
as nephrology when relevant.
259
The literature regarding the appropriate role for RN in
localized disease has evolved substantially yet still
remains controversial in many aspects.
259
Almost all
studies in this domain are retrospective and
observational, and definitive conclusions regarding
comparative efficacy of PN versus RN often cannot be
drawn.
65,204
The only prospective, randomized trial of PN
versus RN was in patients with clinically localized
tumors 5 cm or smaller and demonstrated equivalent
oncologic outcomes.
268
This trial also failed to
demonstrate an overall survival benefit for PN over RN,
and while it can be criticized for a number of flaws, this
is still provocative data suggesting that the survival
benefits of PN in an elective setting may not be as
substantial as previously thought.
212
A prospective trial
of RN versus PN in patients with increased oncologic
risk would address these controversies and would likely
prove very informative.
259
Until this is done, oncologic
and functional considerations and perioperative risks
must be carefully weighed during individualized patient
counseling.
269-271
In select patients RMB may be helpful
for risk stratification, and nuclear renal scan or
differential parenchymal volume analysis
272
to provide
split renal function can also be considered.
200
Surgical Principles
20. For patients who are undergoing surgical
excision of a renal mass with clinically
concerning regional lymphadenopathy,
clinicians should perform a lymph node
dissection including all clinically positive
nodes for staging purposes. (Expert Opinion)
If suspicious lymphadenopathy is identified on imaging
or during surgical exploration, a lymph node dissection
(LND) should be performed with removal of all clinically
evident nodes, if feasible, primarily for staging and
prognostic purposes.
273,274
In a prospective study by
Blom and colleagues, 772 patients with cT1-T3N0M0
RCC were randomized to RN plus LND versus RN
alone.
275
Fifty-one patients in the RN plus LND group
had palpable nodes and 10 (19.6%) were N+ on final
pathology. For patients in this cohort without palpable
nodes only 4/311 (1.3%) were pN+. Overall, only 4%
of patients in the RN plus LND cohort had pN+ disease.
Cancer-specific and overall survival rates were nearly
identical in the RN plus LND and RN alone cohorts. Data
from this study and others have contributed to strong
consensus that LND need not be performed routinely in
patients with localized kidney cancer and clinically
negative nodes.
273-275
Other investigators have studied risk factors for LN
involvement in patients undergoing nephrectomy and
have found that large primary tumor (>10 cm), clinical
stage T3/T4, high tumor grade (Fuhrman grade 3 or 4),
sarcomatoid features, and histologic tumor necrosis all
correlate with increased incidence of pN+ disease.
273
Patients with 2 or more of these risk factors were found
to be at substantially increased risk of nodal
involvement (>40%), and prospective evaluation has
confirmed these findings. Hence, selective performance
of LND should be considered at the time of renal cancer
surgery.
273
However, this is primarily for staging
purposes, as recent studies have been unable to
confirm a survival benefit for lymph node dissection
among patients undergoing RN for non-metastatic RCC.
276-279
If lymph node involvement is confirmed on final
pathology, adjuvant therapy and medical oncology
consultation should be considered (See Statement 24
for specific recommendations regarding this issue).
21. For patients who are undergoing surgical
excision of a renal mass, clinicians should
perform adrenalectomy if imaging and/or
intraoperative findings suggest metastasis or
direct invasion of the adrenal gland. (Clinical
Principle)
Adrenal involvement with RCC is a poor prognostic
finding and fortunately relatively uncommon outside of
the advanced disease setting.
274,280
In the more recent
revisions of the AJCC TNM classification scheme,
adrenal involvement with RCC was upstaged to pT4 if
due to contiguous involvement and pM+ otherwise,
reflecting likely hematogenous dissemination.
54
If
adrenal involvement is confirmed on final pathology,
adjuvant therapies and medical oncology consultation
should be considered (see statement 24).
Several studies have shown that occult adrenal
involvement is uncommon in patients with clinically
localized kidney cancer, and the adrenal gland can be
spared in this setting without compromising oncologic
outcomes.
274,281,282
Adrenalectomy should be performed
if preoperative imaging or intraoperative inspection
suggests metastasis or adrenal enlargement. In this
setting, adrenalectomy has important prognostic utility
and may occasionally have therapeutic potential.
274
The
one exception to this is when the patient has a well-
characterized non-functioning adenoma, which may not
mandate surgical excision.
If locally advanced features are identified
preoperatively or during exploration, adrenalectomy
should be considered if the gland is in close proximity
to tumor. However, the adrenal may be spared in this
setting if the contralateral adrenal gland is absent and
the ipsilateral gland demonstrates normal morphology
and no malignant involvement.
274
22. In patients undergoing surgical excision of a
renal mass, a minimally invasive approach
should be considered when it would not
compromise oncologic, functional, and
perioperative outcomes. (Expert Opinion)
Minimally invasive techniques have permeated surgical
practice with the hope of maintaining the oncological
efficacy of open surgery while reducing its morbidity.
Multiple studies demonstrate recuperative and cosmetic
advantages to minimally invasive RN in comparison to
open surgery.
283-285
Laparoscopic and robotic PN have
demonstrated equivalent surgical margin status and
oncological outcomes when compared to open surgery
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

28
in well-selected patients.
286-288
The high rate of
percutaneous TA, relative to surgically performed
ablation, may explain the favorable perioperative
outcome and harm profile associated with these
treatment options.
204
While minimally-invasive
approaches have also been reported in increasingly
complex indications (large renal masses, renal vein
thrombi and patients with solitary kidneys),
289-293
patient safety and adherence to prior guideline
statements regarding oncologic outcomes, indications
for nephron sparing surgery, and preservation of renal
function should be prioritized relative to the choice of
surgical access approach.
The current data suggest that the benefits of minimally
invasive surgery are realized in the short-term,
perioperative period and are equivalent to open surgery
with intermediate- and long-term follow-up.
294-296
The
limited quality-of-life data that exist in this realm fail to
demonstrate clinically significant differences in health
related quality of life among patients undergoing
laparoscopic and open nephrectomy.
297
While cost-
effectiveness remains unanswered due to limitations of
the data and considerations of long-term surveillance;
the potential increase in costs related to certain
minimally invasive approaches may be balanced with
shorter hospital stays and earlier convalescence.
298-302
Ultimately, the decision for management strategy—RN,
PN, or TA—should be made irrespective of approach
available and clinicians should employ minimally
invasive approaches only when oncological, functional,
and perioperative outcomes are unlikely to be
compromised.
Other Considerations
23. Pathologic evaluation of the adjacent renal
parenchyma should be performed and
recorded after PN or RN to assess for possible
intrinsic renal disease, particularly for
patients with CKD or risk factors for
developing CKD. (Clinical Principle)
Proper evaluation of the non-neoplastic kidney disease
is infrequently performed or reported
303
but is essential
to achieve optimal patient management. Given that
diabetes and hypertension are independent risk factors
for RCC, diabetic nephropathy and hypertensive
nephropathy are found in 8-20% and at least 14% of
tumor nephrectomies, respectively.
144-146
Recognizing
this general deficiency, the College of American
Pathologists established a requirement that pathologic
evaluation of the renal parenchyma for possible
nephrologic disease should be included in all synoptic
reports for kidney cancer.
304
Additional gains in clinical
outcomes may be achieved with improved identification
and management of non-neoplastic renal diseases. In
patients with more significant CKD, particularly those
with significant proteinuria, the urologist may elect to
submit additional specimens of normal parenchyma for
a formal pathologic medical renal evaluation which may
include adjunct testing. In these cases, the urologist
should communicate directly with the pathologist to
optimize testing and the additional information
obtained.
24. Clinicians should consider referral to medical
oncology whenever there is concern for
potential clinical metastasis or incompletely
resected disease (macroscopic positive margin
or gross residual disease). Patients with high-
risk or locally advanced, fully resected renal
cancers should be counselled about the risks/
benefits of adjuvant therapy and encouraged
to participate in adjuvant clinical trials,
facilitated by medical oncology consultation
when needed. (Clinical Principle)
Systemic therapies for metastatic RCC continue to
expand rapidly through the use of targeted therapies,
new generation immunotherapies, combination and
sequential therapies and a broad array of therapeutics
currently in clinical trials. Overall response rates, cancer
-specific and overall survival continue to improve, albeit
with associated toxicities.
305-307
Decisions regarding
when to begin systemic therapy and which therapies to
use in the first and second line are complex and
evolving quickly. Risk stratification using the IMDC and
MSKCC criteria guide initial therapeutic choices and
should be made in consultation with an experienced
medical oncologist.
181,308
Given the success of systemic therapies for metastatic
disease, the role of tyrosine kinase inhibitors and
immunotherapies have been and are being tested in the
adjuvant setting.
309
Multiple nomograms and algorithms
are available to predict recurrence risks and guide
eligibility for adjuvant kidney cancer trials.
310
Clinicians
may access these tools as on-line calculators for point
of care patient counseling. Eligibility and radiographic
assessment for adjuvant clinical trials in kidney cancer
continue to be refined.
311
In 2017, the FDA approved
sunitinib malate as the only therapy for the adjuvant
treatment of adult patients at high risk of recurrent RCC
following resection based on a multicenter, double
blinded placebo controlled trial (S-TRAC) in 615
patients who were randomized to receive either 50mg
of sunitinib malate once daily for 4 weeks on then two
weeks off, or placebo.
312
The study met its primary
endpoint demonstrating an improvement in disease-
free survival; however, significant differences in overall
survival were not observed.
313
While the current standard of care for patients with
fully resected renal cancers remains close clinical and
radiographic observation, patients with a high risk of
recurrence should be counseled regarding systemic
adjuvant options and/or considered for enrollment into
adjuvant clinical trials, facilitated by medical oncology
consultation when needed.
Thermal Ablation (TA)
25. Clinicians should consider TA as an alternate
approach for the management of cT1a solid
renal masses <3 cm in size. For patients who
elect TA, a percutaneous technique is
preferred over a surgical approach whenever
feasible to minimize morbidity. (Moderate
Recommendation; Evidence Level: Grade C)
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

29
The literature regarding TA for localized renal masses
has further matured allowing for a more meaningful
assessment of oncologic outcomes. Follow-up in some
TA studies has now reached 5 years or more and
thereby facilitates a more robust comparison of TA with
surgical excision.
211,247
Results with TA are particularly
encouraging for smaller renal masses (<3 cm) making
it a reasonable alternate approach in this setting. The
recent AHRQ meta-analysis demonstrated comparable
metastasis-free survival for PN and TA
65
, and analysis
of population-based (SEER) and institutional studies
demonstrated median 5-year cancer-specific survival
rates of 100% (range 97-100%) and 94% (range 92-
97%) for PN and TA, respectively.
However, local recurrence-free survival is generally
reported as favoring surgical extirpation. In the recently
updated AHRQ meta-analysis of studies comparing PN
and TA, the risk ratio for local recurrence was 0.55
(95% CI: 0.33-0.91) in favor of PN (Figure 5, see
Statement 14).
65
Median local recurrence-free survival
across the studies was 99.5% for PN and 93.9% for TA.
Since the morbidity of repeat ablation, particularly
percutaneous treatment, is generally low, local
recurrences may often be salvaged with repeat TA.
When considering such salvage attempts in addition to
the initial ablation, the AHRQ meta-analysis reported no
statistical difference in the risk ratio for local recurrence
comparing PN and TA (RR 0.97; 95% CI: 0.47-2.00,
Figure 6).
65
It should be noted; however, that this
analysis was limited by inclusion of only three TA
studies,
314-316
one of which did not report any
recurrences in either group
316
making precise estimates
of recurrence risk impossible. Experience with TA of
cystic renal tumors is limited given concerns for
possible tumor seeding and inhomogeneous distribution
of thermal energy. It is the Panel’s opinion that routine
consideration of TA for cystic lesions requires further
investigation.
Single institution TA studies have optimized therapeutic
efficacy by improving patient selection. Most studies
suggest that increasing tumor diameter is the key
predictive factor, as it has been associated with greater
likelihood of incomplete ablation and local recurrence.
For cryoablation, Tanagho et al.
317
reported that tumor
size > 2.5 cm was the sole factor predictive of local
recurrence on multivariate analysis. Using RFA, Gervais
and colleagues
318
reported 100% effectiveness for
tumors < 3 cm and 81% for tumors larger than 3 cm.
Similarly, Best et al.
319
demonstrated 5-year overall
disease-free survival of 95% for RFA of tumors < 3.0
cm compared to 79% for tumors larger than 3.0 cm.
Although some institutional series advocate TA for
larger tumors, it has been acknowledged that the risk
of complications, in particular renal tumor fracture and
hemorrhage, is higher when treating tumors greater
than 3 cm.
320-322
Thus the panel felt that TA should
optimally be reserved for smaller tumors less than 3 cm
in size unless patient co-morbidities or other factors
dictate otherwise.
Preservation of renal function after treatment is an
important goal in the management of smaller renal
masses, particularly in patients with pre-existent CKD.
As with PN, TA minimizes parenchymal loss and
improves long-term renal function compared to RN. The
AHRQ meta-analysis demonstrated that patients
undergoing TA have similar renal functional outcomes
to those undergoing PN.
65
TA also has a favorable
morbidity profile in comparison to extirpative surgery.
Transfusion rates, length of hospital stay, and
conversion to RN all favor TA over PN.
65
Minor and
major Clavien complication rates do not differ
significantly between TA and PN.
65
Both percutaneous and laparoscopic approaches to TA
have similar efficacy.
323-326
However, the percutaneous
approach is associated with shorter procedure time,
quicker recovery, and lower narcotic requirements and
should be the preferred approach to TA. For instance,
Bandi and colleagues reported that percutaneous
cryoablation was associated with significantly reduced
anesthesia time (148 versus 247 minutes), shorter
mean hospital stay (1.1 versus 2.5 days), and shorter
time to complete recovery (13.5 versus 27.5 days)
when compared to laparoscopic cryoablation.
327
Many of
these considerations translate to an economic
advantage for the percutaneous approach. Hinshaw and
colleagues demonstrated 40% lower hospital charges
for percutaneous cryoablation compared to laparoscopic
cryoablation, and Castle et al. reported that total costs
for percutaneous RFA were over 50% lower than for
laparoscopic RFA.
301,323
Tumor location and complexity also play an important
role in selection for TA. Completely intrarenal lesions or
those immediately adjacent to the sinus or hilum are
more difficult to treat effectively by TA. Percutaneous
displacement techniques such as the use of fluid (hydro
-dissection), carbon dioxide, or spacer balloons
frequently enable separation of adjacent structures
from the anticipated zone of ablation, rendering many
cases suitable for percutaneous TA. A laparoscopic
approach is seldom needed except for occasional cases
in which adhesions prevent displacement of adjacent
structures or when the collecting system is at risk for
serious injury even with thermo-protective maneuvers
such as pyeloperfusion.
326
In such cases, laparoscopic
TA or PN can be considered.
26. Both radiofrequency ablation (RFA) and
cryoablation may be offered as options for
patients who elect TA. (Conditional
Recommendation; Evidence Level: Grade C)
There are no randomized studies directly comparing
cryoablation to RFA. Current retrospective comparisons
are limited by variability in patient selection, tumor size
and location, technique, and laparoscopic or
percutaneous approach. Two large single institution
studies with significant experience with both
cryoablation and RFA have reported comparable
oncologic outcomes (local recurrence-free survival and
cancer-specific survival), impact on renal function, and
complication rates for the two modalities.
328,329
Two
meta-analyses of the literature have confirmed no
significant differences between cryoablation and RFA in
treatment outcomes as defined by complications,
metastatic progression, or cancer-specific
survival.
75,85,330
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

30
Optimal TA requires an understanding of the
mechanism of action for each technique and
appropriate ablation monitoring. RFA utilizes high
frequency alternating current (460-500 kHz) to induce
ion agitation and frictional heating in adjacent
tissue.
331,332
This can be achieved through two types of
radiofrequency generator systems: a temperature-
based system, which drives the current to reach a
target temperature, or impedance-based systems,
which continue ablation until a predetermined
impedance level is reached.
16,331,332
RFA systems utilize
either single or multi-tined electrodes, which are
designed to optimize tissue volume ablation.
331,332
Impedance-based systems apply algorithmic gradual
increases in electrical current while monitoring for rapid
impedance changes that indicate tissue charring near
the electrode. Meta-analysis has demonstrated
reproducible outcomes for ablation of renal masses and
no superiority of either temperature or impedance-
based RFA.
333
Cryoablation systems leverage the Joule Thompson
effect to generate lethal temperatures below -20 to -40
°C, resulting in coagulative tissue necrosis.
332,334,335
The
volume of lethal temperature generated during
cryoablation is regulated by the duration of freezing,
number of freeze cycles, size and number of
cryoprobes, and local tissue interactions.
332,334-337
Woolley et al. showed in a dog model that larger
volumes of renal tissue necrosis result from a double
freeze compared to a single freeze. They found no
difference in volumes of necrosis between active and
passive thawing between the freezing cycles. However,
active thawing saves time.
336
Thus, a commonly used
protocol for renal tumor ablation is termed “10-8-10”,
and consists of two 10 minute freezing cycles separated
by an 8 minute active thawing cycle. Monitoring the
progress of cryoablation is done through real time
imaging of the iceball. Complete treatment of a tumor
requires that the iceball extend beyond the tumor
because the peripheral leading edge of the iceball is at
sub-lethal temperatures, and the iceball thus provides
an overestimate of the zone of ablation.
334,335
Lethal
temperatures are reached approximately 5 mm from
the periphery of the iceball.
332,334,335
RFA and cryoablation differ in how to ensure complete
coverage for larger or irregular tumors. For small
tumors optimally shaped for a given electrode type, a
single RFA application may be sufficient to create a
zone of ablation that covers the tumor. For irregularly
shaped tumors, larger tumors, and/or tumors where
the electrode is not optimally centered in the tumor,
multiple overlapping ablations may be required with
electrode repositioning between ablations to adequately
treat the entire tumor. In contrast to RFA, where
sequential overlapping ablations may be required,
cryoablation allows simultaneous activation of multiple
cryoprobes in the synergistic creation of an iceball that
is larger than the simple additive effect of each
cryoprobe.
334,335,337
Thus, treatment planning involves
choosing the correct number and size of cryoprobes as
well as their relative distribution within a renal tumor in
order to create a zone of lethal ice that covers the
entire tumor.
27. A RMB should be performed prior to
(preferred) or at the time of ablation to
provide pathologic diagnosis and guide
subsequent surveillance. (Expert Opinion)
Although solid, enhancing renal masses are most often
RCC, the differential diagnosis also includes benign
tumors, such as oncocytoma and AML, non-RCC
malignancies, and metastatic lesions. TA by its nature
will lead to tissue necrosis and therefore will not allow
clinicians to acquire diagnostic tissue after ablation has
been performed. A diagnostic RMB prior to TA is
therefore the only realistic opportunity to render a
diagnosis in patients who elect this management
strategy. Notwithstanding most patients’ desire to know
the histology of their tumor, failure to make such a
diagnosis could create significant challenges. These
include difficulty determining the intensity of
surveillance, which might be abbreviated or tailored for
patients who have a benign or indolent lesion.
338
In
addition, emerging evidence suggests that RCC subtype
may impact sensitivity to thermal injury and thereby
treatment success and recurrence risk.
339
Diagnosing a
metastatic lesion may significantly impact treatment or
surveillance for patients with other known
malignancies. Finally, should the patient develop a
recurrence after TA, particularly at a distant site,
knowledge of the primary tumor type could significantly
impact treatment decisions.
For all of these reasons, RMB prior to or concurrent with
TA is strongly advised. Performing RMB prior to TA as a
separate procedure may facilitate more rational
counseling and avoid treatment of benign tumors,
which may be particularly advantageous for patients in
whom the risk of TA may be increased due to
challenging tumor size and location, or for patients with
marginal renal function.
340,341
However, in many cases
RMB as a separate procedure can increase the risk and
cost associated with the TA management strategy.
Therefore, decisions about timing of RMB relative to TA
should be made on an individualized basis.
28. Counseling about TA should include
information regarding an increased likelihood
of tumor persistence or local recurrence after
primary TA relative to surgical excision, which
may be addressed with repeat ablation if
further intervention is elected. (Strong
Recommendation; Evidence Level: Grade B)
There are no prospective, randomized trials that
directly compare local recurrence-free survival (LRFS)
after TA to either RN or PN. The AHRQ meta-analysis
identified 14 retrospective studies (3,916 total patients)
that compared LRFS between TA and PN, while only two
studies (217 patients) compared TA to RN. The formal
analysis was updated and prioritized the limited number
of TA studies with longer follow-up (48 ± 12) to provide
a more meaningful comparison. Local recurrence was
significantly less common with PN when compared to
TA when only the primary ablation was considered (RR
0.55, 95% CI 0.33-091; Figure 5).
65
This corresponded
to local control rates for primary TA in the range of 85-
95% (interquartile range) compared to 97-100% for PN
across studies (Figure 5, see Statement 14). Patients
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

31
should be informed of these differences during
counseling about the relative merits and limitations of
TA. However, when the meta-analysis allowed for a
salvage or secondary ablation, no difference in local
control was noted (RR 0.97, 95% CI 0.47-2.00)(Figure
6).
65
A small minority of patients with local recurrence
after TA are not candidates for salvage TA due to tumor
progression and may require surgical salvage. In this
setting, post-ablation fibrosis may present substantial
challenges, and a minimally invasive approach may not
be feasible. However, PN is typically achievable even in
this salvage setting, although experience with this
scenario can be of considerable value.
342,343
There was
insufficient evidence to compare LRFS rates for TA
versus surgical extirpation based on the type of
ablation (RFA or cryoablation) or approach to ablation
(laparoscopic or percutaneous).
Active Surveillance (AS)
The decision to embark on a course of AS or expectant
management rather than treatment in the setting of a
localized renal mass presumed to be a renal cancer
requires thoughtful consideration by both the patient
and the physician. In making the decision, an objective
baseline evaluation of patient, tumor, and treatment-
related factors should be undertaken (Figure 7). This
should include formal decision-making tools whenever
possible leading to a well communicated risk-benefit
analysis unique to the individual patient’s
circumstances.
110,344-347
The shared decision-making
process should be consistent with the patient’s inherent
preferences and tolerance of uncertainty.
348
High level data regarding the optimal frequency and
preferred imaging modalities for renal mass
surveillance are lacking. Therefore, at the time of the
initial baseline assessment and during subsequent re-
assessments, the clinician should estimate how to best
achieve the goals of (1) preventing stage progression,
(2) maintaining renal function and (3) avoiding the
potential risks of treatment when it is unlikely to
provide an oncologic or survival benefit. At the onset of
AS, the clinician should request and evaluate prior
abdominal imaging that may demonstrate the existence
of the renal mass at an earlier time point to assess
growth rate or changes in clinical stage. Next, patients
placed on a program of non-intervention should be
considered for either AS or expectant management
(observation or watchful waiting) (Figure 7).
AS is most appropriate for patients in whom the
anticipated net benefit of AS is modest to significant
when compared to treatment. Excluded from this track
are patients who are reasonable candidates for
intervention if tumor size, infiltrative appearance,
interval growth, or RMB suggest the potential for cancer
progression, unless they are willing to accept the
associated increase in oncologic risk (see statement 31
and 32 below). Patients with no prior imaging should
have surveillance imaging initially every 3 to 6 months
to assess for interval growth, substantial radiographic
changes in the character of the lesion, or the presence
of rare occult synchronous metastases in the setting of
a small renal mass. The preferred modality is not well
established in the literature, but initial imaging should
preferably consist of contrast-enhanced cross-sectional
imaging. Subsequent imaging may include the same or
when appropriate an abdominal US can be substituted.
Abdominal US (as opposed to retroperitoneal US), may
have the additional benefit of a survey of the
intraabdominal organs for progression. Differences in
tumor dimension measurements between these
different modalities may be significant and should be
interpreted with caution when making treatment
decisions.
80
RMB can be considered for additional risk
stratification for patients with solid masses on AS. For
those with predominantly cystic lesions, RMB should be
avoided.
It is recognized that not all patients on AS will require
the same intensity of surveillance as their tumor
biology, risk calculations and tradeoffs, and personal
objectives may differ. Some patients may therefore
require more intensive AS while others require less
intensive AS. The decision as to the frequency and
imaging modality must therefore be customized and
informed by robust communication focusing on goals,
risks and triggers for intervention. RMB can be a helpful
adjunct to guide these clinical decisions (see statement
10). However, even when RMB suggests the tumor is
benign, the predictive value of a core biopsy is
imperfect due to tumor heterogeneity and the
possibility of collision tumors.
192,193
Currently there are
insufficient data to recommend that all patients with
benign RMBs can be advised that they no longer need
follow-up imaging. Judicious surveillance in appropriate
patients with benign appearing RMBs remains a prudent
strategy.
Expectant management (observation) is appropriate in
patients in whom treatment poses an unacceptably high
periprocedural or renal functional risk than surveillance.
In this setting, the use of abdominal US of the
retroperitoneal and intraperitoneal organs can be
performed more frequently than formal contrast based
cross-sectional imaging to screen for stage progression
which may trigger systemic or palliative therapy in the
appropriately selected patient.
Regardless of the intensity of surveillance, chest
imaging with plain radiography (CXR) is warranted
annually or if intervention triggers are encountered or
symptoms arise. The intensity of surveillance can be
attenuated if the renal mass exhibits slow growth
kinetics, is noted to be radiographically stable or if the
patient’s medical condition deteriorates. In cases such
as this, patients can cross over between AS and
expected management (observation) based on
changing risk profiles, performance status, absolute
tumor size, tumor growth kinetics, stage progression or
other recalibration triggers for possible
intervention.
349,350
While no level 1 data exist that
define these triggers precisely, they should generally be
based on changes in tumor-based risk (absolute size >
3cm, median growth rate in excess of 5mm/year, or
stage migration) or patient-based risks (co-morbidities)
with continual objective reassessments to include the
use of RMB when appropriate.
349,350
Published data
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

32
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Figure 7. Algorithm for AS or expectant management of localized renal masses suspicious for
malignancy.

33
demonstrate that in most instances, judicious delayed
intervention for localized stage I renal masses remains
effective.
159,349-354
The key to successful AS of a localized renal mass
remains thoughtful and recurrent reassessments and
robust communication in partnership with the patient
and his/her caregivers. Prospective trials, ideally
randomized, of AS versus treatment, with improved
reporting and more extended follow-up, should be
prioritized to provide higher quality data about
oncologic, functional and survival outcomes.
29. For patients with a solid renal mass < 2cm, or
those that are complex but predominantly
cystic, clinicians may elect AS with potential
for delayed intervention for initial
management. (Conditional Recommendation;
Evidence Level: Grade C)
AS appears to be a safe and effective option for
selected patients who have been properly informed of
the risks and benefits of each management strategy. In
the published AS literature, in which patients were
primarily greater than 70 years old, tumor size
averaged approximately 2 cm, and follow-up ranged
from 12-36 months, cancer-specific and metastasis-free
survival rates were 98-100%.
349,350
When the oncologic
risks are particularly low and the pathology of the
lesion is uncertain, (e.g., tumors < 2 cm), AS with
potential delayed intervention is an acceptable option
for the initial management of all patients, not just those
with limited life expectancy or poor performance status.
More recent studies have demonstrated that complex
cystic masses, particularly Bosniak 3 category lesions
and those that are predominantly cystic, also often
have indolent tumor biology and favorable outcomes on
AS.
152-154
Repeat imaging in 3-6 months to assess for interval
growth or substantial radiographic changes in the
character of the lesion will provide an additional
opportunity to intervene if treatment is deemed
appropriate (Figure 7). Tumor factors that should
prompt consideration for treatment include tumor size
>3 cm, median growth rate >5 mm per year,
infiltrative appearance, clinical stage migration, or
aggressive histology on RMB (Table 6).
349,350
30. For patients with a solid or Bosniak 3/4
complex cystic renal mass, clinicians should
prioritize AS/expectant management when
the anticipated risk of intervention or
competing risks of death outweigh the
potential oncologic benefits of active
treatment. In asymptomatic patients, the
panel recommends periodic clinical
surveillance and/or imaging based on shared
decision making. (Clinical Principle)
It is recognized that surveillance of a likely (or
confirmed) renal malignancy poses some risk of
progression and death from disease. However, for
patients with limited life expectancy, those who
represent unacceptable surgical risks, or those who
potentially face ESRD and initiation of HD, surveillance/
expectant management is a rational non-interventional
nephron-sparing strategy that can save the patient
potentially serious perioperative risks of intervention.
Many localized small renal masses are relatively
indolent at inception and of less clinical significance
compared to other competing comorbidities in
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Table 6. Patient and tumor related factors favoring AS/Expectant Management versus Intervention
Patient-related factors Tumor factors
Favor AS/ Expectant
Management
Elderly
Life expectancy < 5 years
High calculated comorbidities
Excessive perioperative risk
362
Poor functional status
Marginal renal function (>CKD3b)
Patient preference to avoid treat-
ment risks
Maximal tumor diameter < 3 cm
Non-infiltrative on imaging
Intralesional fat suggestive of an AML
Favorable histology (if RMB per-
formed)
Predominantly cystic features
Median tumor growth < 5 mm per
year
Favor Intervention
Young
Life expectancy > 5 years
Healthy: low calculated comorbid-
ity
Acceptable perioperative risk
Good functional status
Anticipate adequate renal function
following intervention
Patient preference for treatment
Maximal tumor diameter > 3 cm
Infiltrative on imaging
Suspicion for advanced T stage
Unfavorable histology (if RMB per-
formed)
Median tumor growth > 5 mm per
year

34
populations at risk.
9,150,355
Thus, in some patients, the
competing risks of death from comorbidities (e.g.,
cardiovascular disease, chronic obstructive pulmonary
disease, or CKD) outweigh the potential oncological and
survival impact of a localized small renal mass. Hence,
expectant management (observation) with serial
imaging is a preferred initial management option for
such patients (Figure 7).
The decision to prioritize observation when the
anticipated risk of intervention or competing risks of
death outweigh the potential oncologic benefits of
active treatment should jointly involve the physician,
the patient and caregivers. Steps to ensure that
patients and loved ones are well informed are
important in engaging them as active participants in
this strategy. Studies show a link between good
communication between patient and physician and
eventual care outcomes.
348
Clinicians should orient and
subsequently re-orient patients regarding AS, and also
consider having both print and online resources
available to facilitate patient education. Patients and
caregivers should be included in the discussions and
encouraged to keep good records, noting improvements
or diminishments in symptoms or health conditions
once observation begins.
Discussions regarding a planned course of observation
should occur with the same depth and intensity of those
regarding treatment. Patients should experience a
supportive, empowered environment. The clinician
should share details of test results and take the time to
ensure the patient understands the dynamic context in
which the information is being provided. To ensure
comprehension, clinicians should speak slowly, avoid
overly technical terminology, and consider providing a
printed summary of key elements of the discussion.
Having the patient verbally reiterate key information
should also be considered to ensure that the goals of
AS/expectant management are understood.
31. For patients with a solid or Bosniak 3/4
complex cystic renal mass in whom the risk/
benefit analysis for treatment is equivocal and
who prefer AS, clinicians should consider RMB
(if the mass is solid or has solid components)
for further oncologic risk stratification. Repeat
cross-sectional imaging should be obtained
approximately 3-6 months later to assess for
interval growth. Periodic clinical/imaging
surveillance can then be based on growth rate
and shared decision-making with intervention
recommended if substantial interval growth is
observed or if other clinical/imaging findings
suggest that the risk/benefit analysis is no
longer equivocal or favorable for continued
AS. (Expert Opinion)
For patients with clinical T1 solid and complex cystic
renal masses, AS appears to be a safe and effective
option for selected patients who have been properly
informed of the risks and benefits of each management
strategy. In patients for whom the risk/benefit analysis
for treatment is equivocal and who prefer AS, diligent
follow-up at 3-6 months is recommended. Patients
should be informed that the risks of metastatic
progression in the short-term (median 24-36 months)
are low (<3%), but not zero.
78,80,159,349,350,356
Absolute
tumor size, tumor complexity, infiltrative appearance
and median growth may all predict progression (Table
6).
349,350
An initial period of AS with delayed intervention has
been shown to be associated with acceptable oncologic
outcomes, albeit with a small risk of upstaging in
selected patients.
78,159,349-351,355,356,361
Absolute triggers
for intervention have not been prospectively defined.
The decision to intervene is complex and based on
multiple risks and tradeoffs.
When calculating growth rate as a trigger for
intervention, the clinician should recognize that normal
variations in maximal tumor diameter and volume
calculations exist between imaging modalities and that
interreader variability may be significant. Moreover,
spider plots of tumor growth rates suggest that
localized renal masses under AS do not always exhibit
linear growth but rather may undergo episodic and/or
Gomertzian (sigmoid-shaped) growth patterns.
159,349,357-
360
Good clinical practice is for the urologist to review
films in sequence, preferably comparing similar
modalities, contrast phases and images over time to
calculate a median growth if this is the primary trigger
for intervention.
Whereas histology may improve stratification for
success or failure of AS, clinicians should consider RMB
in patients with an equivocal clinical risk/benefit
analysis who prefer AS.
200
Pursuing AS in such patients
without tissue confirmation will potentially expose them
to ongoing anxiety associated with an uncertain
diagnosis. Similarly, the knowledge of higher risk
histopathology may recalibrate the AS versus treatment
risk/benefit analysis. Please refer to guideline
statements 10 and 13, which include pertinent details
regarding the processes, risks and performance
characteristics of RMB and further considerations for
patient counseling.
32. For patients with a solid or Bosniak 3/4
complex cystic renal mass in whom the
anticipated oncologic benefits of intervention
outweigh the risks of treatment and
competing risks of death, clinicians should
recommend intervention. AS with potential for
delayed intervention may be pursued only if
the patient understands and is willing to
accept the associated oncologic risks. In this
setting, clinicians should encourage RMB (if
the mass is predominantly solid) for additional
risk stratification. If the patient continues to
prefer AS, close clinical and cross-sectional
imaging surveillance with periodic
reassessment and counseling should be
recommended. (Moderate Recommendation;
Evidence Level: Grade C)
Despite significant advances in the systemic
management of advanced kidney cancer, metastatic
RCC of any histology remains incurable. For this reason,
in patients in whom the oncologic benefits of
intervention outweigh the risks of treatment and
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

35
competing risks of death, clinicians should recommend
a proactive approach. Factors which favor intervention
may be patient-related or tumor-related (Table 6).
Patients with relatively low co-morbidity and an
anticipated life expectancy >5 years should be
prioritized for treatment, particularly when the renal
mass is >3 cm and/or demonstrates median growth of
> 5 mm/year. In these settings, AS may place the
patient at increased risk of local and distant
progression, and treatment may thus provide an
oncologic and survival advantage.
262,356,361
Increasing
tumor size correlates with increased incidence of high
nuclear grade, less favorable histology, and locally-
advanced features.
9,150
Infiltrative appearance on
imaging also suggests high nuclear grade and/or poorly
differentiated elements, such as sarcomatoid
features.
150,263
Median growth rates exceeding 5mm/
year are indicative of oncologically-active tumors and
have been associated with tumor progression and
metastasis.
159,349,350
In these patients, the decision to
pursue RMB should be individualized.
FOLLOW-UP AFTER INTERVENTION
General Principles
33. Clinicians coordinating follow-up for patients
who have undergone intervention for a renal
mass should discuss the implications of stage,
grade, and histology including the risks of
recurrence and possible sequelae of
treatment. Patients with pathologically-
proven benign renal masses should undergo
occasional clinical evaluation and laboratory
testing for sequelae of treatment but most do
not require routine periodic imaging. (Expert
Opinion)
After intervention, providers should discuss with
patients the information available on the pathology
report, including tumor histology, stage, grade, and
surgical margin status, as well as risk of recurrence
based on established nomograms/calculators. In
addition, post-procedural renal function and nephrology
referral should be discussed, as needed.
Given the reduced oncologic potential, routine
postoperative imaging is not required in most patients
after surgical treatment for a benign renal mass.
However, such patients should undergo at least one
postoperative visit to assess patient recovery and
laboratory testing to assess renal function. Further
surveillance for adverse sequelae of treatment, such as
progressive decline in renal function, may also be
required selectively. In addition, patients who have
only had a biopsy without definitive management, may
carry a small risk of a missed malignancy and should be
considered for attenuated surveillance.
34. Patients with treated malignant renal masses
should undergo periodic medical history,
physical examination, laboratory studies, and
imaging directed at detecting signs and
symptoms of metastatic spread and/or local
recurrence as well as evaluation for possible
sequelae of treatment. (Clinical Principle)
Interval patient history and physical examination are an
integral part of medical care, offering the opportunity to
yield critical information regarding the presence of
disease recurrence or adverse events related to
treatment effects. A myriad of signs and symptoms,
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Figure 6. Meta-analysis of local recurrence-free survival for PN versus combined efficacy of primary
and/or repeat TA among studies with follow-up of 48 months ± 12 months.
65
Abbreviations: CI, confidence interval; IV, inverse variance; PN, partial nephrectomy; TA, thermal ablation.
Note: Total patients is defined as total patients with biopsy proven RCC treated with each modality. Events refer to
number of patients with local recurrence.

36
both organ-specific and systemic, including weight loss,
night sweats, shortness of breath, pleuritic chest pain,
hemoptysis, epistaxis, dermatologic involvement,
musculoskeletal pain, weakness, or focal neurological
deficits may herald disease recurrence/progression and/
or the development of a complication and serve as an
indication for further investigation. Physical
examination should assessment for masses in the
abdomen/abdominal wall, lymphadenopathy
(supraclavicular, axillary, groin), or lower extremity
edema that might suggest recurrence with IVC
involvement. Specific recommendations for
surveillance abdominal and chest imaging are provided
in statements 43 and 44.
35. Patients with treated malignant renal masses
should have periodic laboratory testing
including serum creatinine, eGFR, and
urinalysis. Other laboratory evaluations (e.g.,
complete blood count, lactate dehydrogenase,
liver function tests, alkaline phosphatase and
calcium level) may be obtained at the
discretion of the clinician or if advanced
disease is suspected. (Expert Opinion)
Please see the renal assessment background sections
for a discussion of the benefits of monitoring renal
function and referral to nephrology. This should include
periodic assessment of serum creatinine levels, eGFR,
and urinalyses to evaluate for proteinuria, hematuria,
or inflammatory changes.
LDH is included in several nomograms where it provides
prognostic information, in particular for patients with
advanced disease.
363,364
However, there are no data
that demonstrate that regular LDH measurements in
the non-metastatic setting improve detection of
metastatic disease, and this test should thus be used
selectively. Although no strong evidence exists for the
use of these laboratory tests in the follow-up of patients
with clinically localized renal cancers, a common-sense
approach dictates that measures of general organ
function are part of routine follow-up for patients who
are diagnosed with cancer.
While elevated pre-operative alkaline phosphates
365
is a
potential prognostic marker for RCC, retrospective
reviews do not demonstrate utility of either bone scan
or alkaline phosphatase in the initial evaluation or
routine follow-up of asymptomatic patients with
RCC.
366,367
36. Patients undergoing follow-up for treated
renal masses with progressive renal
insufficiency or proteinuria should be referred
to nephrology. (Expert Opinion)
The long-term impact of renal dysfunction increases
risks of osteoporosis, anemia, metabolic and
cardiovascular disease, hospitalization and death.
Effective treatment strategies are available to slow the
progression of CKD and reduce cardiovascular risks,
and therefore timely identification of progressive renal
dysfunction and/or proteinuria can provide opportunity
for medical intervention when indicated. The two
formulas for monitoring eGFR commonly reported in the
contemporary literature at this time are the
Modification of Diet in Renal Disease and CKD –
Epidemiology Collaboration (CKD-EPI) equations. Please
refer to the Presentation and Diagnosis section for
additional information.
37. Patients undergoing follow-up for treated
malignant renal masses should only undergo
bone scan if one or more of the following is
present: clinical symptoms such as bone pain,
elevated alkaline phosphatase, or
radiographic findings suggestive of a bony
neoplasm. (Moderate Recommendation;
Evidence Level: Grade C)
Studies that address the utility of an initial bone scan in
the evaluation of patients with of RCC show that,
although bone scan has a reasonable sensitivity and
specificity, the probability of finding bony neoplasms in
the absence of elevated alkaline phosphatase or bone
pain is low.
96-99
As such, the routine use of bone scan in
the absence of bone pain or elevated ALP should not be
pursued. However, with the presence of symptoms and/
or elevated markers, radionuclide bone scan can be a
useful test.
96,368
This recommendation is based on studies indicating
that an elevated alkaline phosphatase or the presence
of clinical symptoms, such as bone pain, raises the
probability of metastatic spread to a level >5%-10%.
Assuming a sensitivity of 94% and a specificity of 86%
with a pre-test probability of 5%, a negative bone scan
would drop the post-test probability below 1%, whereas
a positive test would raise the post-test probability to
26%, likely necessitating further diagnostic evaluation.
In this setting, the Panel judged the benefit to risk/
burden ratio to favor the performance of a bone scan in
the setting of symptoms or elevated alkaline
phosphatase.
369
There are no compelling data that supports the routine
use of bone scan in the follow-up of patients with non-
metastatic disease. This recommendation is based on
studies indicating that in the absence of an elevated
ALP or clinical symptoms, such as bone pain, the
prevalence of bony metastases is very low (<1%).
Routine imaging of these patients would result in a high
rate of false-positive findings necessitating further
burdensome, potentially invasive and resource
intensive studies.
38. Patients undergoing follow-up for treated
malignant renal masses with acute
neurological signs or symptoms should
undergo prompt magnetic resonance imaging
(MRI) or computed tomography (CT) scanning
of the brain and/or spine. (Strong
Recommendation; Evidence Level: Grade A)
This recommendation is based on high diagnostic
accuracy of neurologic cross-sectional (CT or MRI)
imaging to identify or exclude metastases to the brain
and/or spine, in addition to a high prevalence of
underlying management-altering pathology in patients
with these symptoms, including but not limited to
metastatic disease. MRI may be more sensitive than CT
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

37
scan for the detection of small CNS neoplasms. CT may
be used in the setting of acute neurological signs or
symptoms to diagnose abnormalities that require
emergent treatment,
370,371
but MRI is the most sensitive
and specific imaging test for detection of metastatic
neoplasms to the brain.
372
39. For patients undergoing follow-up for treated
malignant renal masses, additional site-
specific imaging can be ordered as warranted
by clinical symptoms suggestive of recurrence
or metastatic spread. Positron emission
tomography (PET) scan should not be
obtained routinely but may be considered
selectively. (Moderate Recommendation;
Evidence Level: Grade C)
Occasionally, patients will present with symptoms that
could be attributed to metastatic disease. These
symptoms may include, but are not limited to, new
onset bone pain, weight loss, anorexia, abdominal
discomfort, asthenia, fatigue, gross hematuria and
lower extremity edema. When patients present with
symptoms that could be attributed to disease
recurrence or metastasis, site-specific imaging should
be obtained, and the modality of imaging (CT, MRI, US,
bone scan, plain films) should be tailored to the specific
presenting symptoms.
PET scan should not be routinely obtained in the follow-
up of patients after RCC treatment, as a review of the
evidence failed to identify studies to conclusively
support a role for FDG-PET in this setting.
373
The main
limitations of FDG are its lack of sensitivity and
specificity for detecting RCC. False positive results can
be seen in postsurgical scarring,
374
and concurrent
infectious or inflammatory processes,
375,376
while false
negative results can be seen with small recurrence
374,375
and can be inherent to PET scanner limited resolution
or close proximity of the recurrence to the collecting
system and urinary tract which routinely lights up on
PET.
374
Well-designed prospective studies on the role of FDG
PET/CT are still needed prior to routine clinical use in
the follow-up of patients with kidney cancer after
definitive treatment. Future roles may exist for PET/CT
with newer imaging agents, such as Zirconium
89
-
girentuximab, which are currently being studied in
prospective trials.
374
40. Patients with findings suggestive of
metastatic renal malignancy should be
evaluated to define the extent of disease and
referred to medical oncology. Surgical
resection or ablative therapies should be
considered in select patients with isolated or
oligo-metastatic disease. (Expert Opinion)
After undergoing a thorough investigation with medical
history, physical examination, laboratory studies, and
imaging, patients with findings suggestive of metastatic
disease should be referred to a medical oncologist for
additional evaluation and management. For
appropriately selected patients with good performance
status and isolated or oligo-metastatic disease, surgery
and ablation should be considered after
multidisciplinary discussion.
378
Complete resection of
solitary or isolated metastases can lead to 5-year
disease-free status in 20-30% of patients with results
varying based on several prognostic factors, including
performance status, time from initial treatment to
metastasis, number and size of metastatic lesions, site
of metastases, and factors reflecting the tumor biology
of the primary lesion, including stage, grade, and
histology.
41. Patients with findings suggesting a new renal
primary or local recurrence of renal
malignancy should undergo metastatic
evaluation including chest and abdominal
imaging. If the new primary or recurrence is
isolated to the ipsilateral kidney and/or
retroperitoneum, a urologist should be
involved in the decision-making process, and
surgical resection or ablative therapies may
be considered. (Expert Opinion)
Local recurrence is defined as any persistent or
recurrent disease present in the treated kidney or
associated renal fossa after initial treatment. Local
recurrence or persistence after TA includes persistent
enhancement of any treated mass, a visually enlarging
neoplasm or new nodularity, or failure of regression in
size of the treated lesion(s), or new satellite or port site
lesions. Patients who are found to have a new renal
primary tumor, or a local recurrence as defined above
should undergo a metastatic evaluation (CT chest and
either CT or MRI abdomen are preferable). Additional
imaging can be obtained as needed. For appropriately
selected patients with good performance status and an
isolated new renal primary tumor or a local recurrence,
surgery or ablation should be considered for definitive
management.
Follow-up After Surgery
42. Clinicians should classify patients who have
been managed with surgery (PN or RN) for a
malignant renal mass into one of the following
risk groups for follow-up:
If final microscopic surgical margins are positive
for cancer, the risk category should be considered
at least one level higher, and increased clinical
vigilance should be exercised. (Expert Opinion)
The literature previously suggested that a variety of
algorithms or nomograms could provide relatively
Low Risk
(LR):
pT1 and Grade 1/2
Intermedi-
ate Risk
(IR):
pT1 and Grade 3/4, or pT2
any Grade
High Risk
(HR):
pT3 any Grade
Very High
Risk (VHR):
pT4 or pN1, or sarcomatoid/
rhabdoid dedifferentiation, or
macroscopic positive margin
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

38
robust and accurate prediction of risk of recurrence
after surgical management of RCC. However, Correa
and colleagues recently studied 8 of these RCC
recurrence models (UISS, SSIGN, Leibovich, Kattan,
MSKCC, Yaycioglu, Karakiewicz, and Cindolo) as applied
to the results of a phase III adjuvant therapy clinical
trial, with direct comparison of what would be predicted
by stage alone.
94
Model performance ranged from a c-
index of 0.556 (UISS) to 0.688 (SSIGN). Most of these
models only marginally outperformed the 2002 TNM
staging system (c-index of 0.60). With these data in
mind, the Panel formulated a simple grouping to keep
risk stratification convenient for routine patient care,
while differentiating risk groups in a clinically
meaningful fashion. The same follow-up schedule
applies to all RCC histologies. Data regarding the risk of
recurrence in each of these cohorts is listed below.
LR: Patients w ith pT1 have a recurrence rate of
9.2%, while patients with Grade 1 and Grade 2 have
recurrence rates of 6.4% and 15.4%, respectively.
379
IR: P atients w ith pT2 have a recurrence rate of
32%, while patients with organ-confined RCC, Grade
3/4 tumors have recurrence rates of approximately 20-
30%. Recurrence rates for Grade 4 tumors may be
higher in certain circumstances (larger tumor or non-
organ confined) and can be considered at least one risk
category higher at physician discretion.
379
VHR: M ost patients w ith pT4 present w ith
metastatic disease at the time of surgery. Of those who
are initially free of disease after surgical resection,
64.7% have disease recurrence (mostly distant alone,
but local often seen too).
380
Patients with nodal
involvement (pN1) who undergo complete surgical
resection have median cancer-specific survival of 2.8
years, with 64.3% dying of RCC after recurrence.
381
In
one study, patients with sarcomatoid dedifferentiation
were found to have a 72% recurrence rate, with a
median time to recurrence of 26.2 months.
382
Over
seventy percent presented with a single site of disease
at time of first recurrence (lung, 45%; local, 25%;
bone, 13%; liver, 13%).
383
In patients with grade 4 non
-metastatic RCC, sarcomatoid dedifferentiation was
associated with an 82% increased cancer-specific
death. Wood and colleagues studied patients with
positive surgical margins after PN and reported a tumor
bed recurrence rate of 15.9% (versus 3% in a matched
control group), indicating the need for closer follow-up
in patients with positive surgical margins after PN. The
risk for patients with macroscopic positive margins is
even higher, as these patients have residual disease
and are very high-risk for developing clinical local
recurrence.
43. Patients managed with surgery (PN or RN) for
a renal malignancy should undergo abdominal
imaging according to Table 1, with CT or MRI
pre- and post-intravenous contrast preferred.
(Moderate Recommendation; Evidence
Strength: Grade C). After 2 years, abdominal
ultrasound (US) alternating with cross-
sectional imaging may be considered in the LR
and IR groups at physician discretion. After 5
years, informed/shared decision-making
should dictate further abdominal imaging.
(Expert Opinion)
Merrill and colleagues studied the difference in survival
outcomes in 78 patients (of 737 surgically treated
patients) presenting with a symptomatic versus
asymptomatic recurrence.
384
Symptomatic recurrences
were associated with a 3-fold increased risk of cancer-
specific mortality. These results were consistent for
both local and systemic recurrences. Other studies
support the notion that the size of a local recurrence is
associated with survival outcomes, underscoring the
potential benefits of routine scheduled postoperative
surveillance for the detection of early recurrences while
still asymptomatic.
385
Beisland and colleagues prospectively followed 312
patients surgically treated for non-metastatic RCC using
a risk-stratified approach (using Leibovich risk
groups).
386
They noted that patients who were
diagnosed with a recurrence during the scheduled
follow-up program experienced longer survival and
were more frequently able to receive tumor-directed
therapy than those who were not. Such studies support
a proactive approach to follow-up after intervention,
which reflects most urologists’ clinical experience.
Duration of follow-up after intervention for RCC has
been controversial. Previous guidelines suggested that
surveillance can be either terminated or strongly
attenuated relatively soon (3-5 years) after surgery.
However, recent studies suggest that 30% of RCC
recurrences are diagnosed beyond 5 years after
surgery. Stewart and colleagues studied an institutional
cohort of 3,651 patients treated surgically for non-
metastatic RCC.
1
Patients were classified based on AUA
risk, and the recurrence detection rates based on
AUA,
387
NCCN 2013 and NCCN 2014 guidelines were
compared.
181
Of note, all 3 guidelines do not offer
scheduled imaging after 5 years from date of surgery.
29.8% of patients experienced cancer recurrence at a
median of 1.9 years after surgery. The authors found
that the 2013 NCCN, 2014 NCCN, and the AUA
guidelines captured 35.9%, 68.2%, and 66.9% of all
recurrences, respectively. Extending surveillance
protocols up to 10 years would have improved
recurrence detection rates to around 90%.
The option to use abdominal US instead of CT or MRI at
physician discretion after 5 years of follow-up is
intended to allow continuous monitoring after 5 years,
while minimizing radiation exposure/cost in the LR and
IR groups.
44. Patients managed with surgery (PN or RN) for
a renal malignancy should undergo chest
imaging (chest x-ray [CXR] for LR and IR; CT
chest preferred for HR and VHR) according to
Table 1. (Moderate Recommendation;
Evidence Strength: Grade C). After 5 years,
informed/shared decision-making discussion
should dictate further chest imaging and CXR
may be utilized instead of chest CT for HR and
VHR (Expert Opinion)
As pulmonary metastases are the most common site of
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

39
renal cancer recurrence, timely detection of recurrent
disease in the chest is optimized by a chest CT, which
can be performed at the same time as the abdominal
imaging and should be prioritized whenever CXR is
equivocal or suspicious. The option to use CXR instead
of chest CT after 5 years of follow up is intended to
allow continuous monitoring after 5 years, while
minimizing radiation exposure/cost in the HR and VHR
groups.
As the utility of adjuvant therapy is still limited, early
detection of metastatic disease is vital for improving
patient outcomes. Chest and abdominal metastases are
usually asymptomatic while small, with symptoms
developing mainly in advanced stages.
388
Early
intervention when surgical resection or ablation is
feasible could improve patient outcomes.
389
Follow-up After TA
45. Patients undergoing ablative procedures with
biopsy that confirmed malignancy or was non-
diagnostic should undergo pre- and post-
contrast cross-sectional abdominal imaging
within 6 months (if not contraindicated).
Subsequent follow-up should be according to
the recommendations for the IR postoperative
protocol (Table 1). (Expert Opinion)
The Panel considers urologists to be the experts in the
evaluation, management and follow-up of both the
small renal mass as well as renal cancer and the
treatment associated complications. Urologists should
be involved in the care of the patient whether or not
they perform the actual procedure. They should be
active partners of interventional radiologists, and
participation in the percutaneous procedure is
encouraged.
This recommendation is based on a 5-10% failure rate
of ablative therapy and places a high value on the early
detection by CT or MRI scans to direct potential
retreatment and successful salvage. Close attention to
overall pattern and morphology, with respect to
growth/shrinkage and nodularity of the neoplasm over
time, as well as contrast enhancement on serial follow-
up scanning is advised. Patients who cannot receive IV
contrast due to renal dysfunction or allergies should still
undergo cross-sectional MRI (preferably, and ideally
contrast-enhanced) or CT scan to assess for regression
of the treated lesion and to monitor for new nodularity
or growth. As previously stated, any growth in the size
of the treated lesion, lack of regression in size of the
lesion over time, new nodularity (in the kidney itself,
the surrounding soft tissue, or the port sites) or
enhancement beyond six months from ablation would
be concerning and should prompt further investigation,
including a biopsy as needed.
Patients who have undergone ablative treatment of
renal tumors are subsequently followed with radiologic
scanning using CT or MRI. Immediate post-procedural
imaging of the ablated tumor generally shows the
treatment bed to be larger than the pre-treatment
tumor size for RFA due to ablation of a peripheral
margin of normal tissue, and for cryoablation due to
extension of the iceball beyond the original tumor
margin. Radiological evolution of cryoablated tumors is
characterized by significant shrinkage and loss of
contrast enhancement on CT. Tumors successfully
treated with RFA demonstrate no IV contrast
enhancement but there is often minimal shrinkage
observed on cross-sectional imaging.
390
On MRI, the
imaging hallmark of successful renal tumor ablation is
lack of tumor enhancement with gadolinium-enhanced
imaging. Rim enhancement, believed to represent
reactive change, may occasionally be seen at early
postprocedural MR scanning after RFA or cryoablation,
which later resolves.
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Table 1: Recommended follow-up schedule after surgery for renal cancer (in months)*
*Follow-up timeline is approximate and allows flexibility to accommodate reasonable patient, caregiver, and in-
stitutional needs. Each follow-up visit should include relevant history, physical examination, laboratory testing,
and abdominal and chest imaging. Overall, 30% of renal cancer recurrences after surgery are diagnosed beyond
60 months.
1
Informed/shared decision-making should guide surveillance decisions beyond 60 months.
Risk 3 6 9 12 18 24 30 36 48 60 72-84 96-120
LR x x x x x x
IR x x x x x x x x
HR x x x x x x x x x x
VHR x x x x x x x x x x x x

40
FUTURE DIRECTIONS
The most promising routes to advance the field in
localized renal cancer include (1) clinical trials, (2)
collaborative quality initiatives, (3) novel diagnostics/
biomarkers, and (4) improved technologies and
systemic therapies. Each of these requires an
unrelenting commitment to continuous clinical
improvement and scientific investigation.
The management of localized renal cancer is an area for
which there is a paucity of randomized clinical trials
(RCT’s). Improving the strength of evidence will require
an increased commitment to clinical trial design,
conduct, and funding. Although our understanding of
the nature and management of this disease continues
to progress, without adequate engagement and
support, our treatment paradigms will likely continue to
be more art than science.
An appropriate companion to RCT’s is the development
of collaborative quality initiatives (CQI’s).
391
Within a
CQI, participating hospitals and providers collect, share,
and analyze data through clinical registries. CQI
participants design and affect changes that improve
outcomes of complex, highly technical areas of care.
392
CQI registries allow for a more robust analysis of the
link between processes and outcomes than can occur
with retrospective single or multi-institutional studies;
particularly as more sensitive and specific diagnostics/
biomarkers are complemented by technologic
advances. Scientific inquiry will continue to provide
fundamental knowledge regarding the biological basis,
inherent risks, and natural history of localized renal
masses such that appropriate trade-offs can be made
when considering optimal management.
Evaluation and Diagnosis
The localized renal mass remains primarily a
radiographic diagnosis. The field of tumor radiomics,
artificial intelligence and molecular imaging promises
393
to improve our ability to discriminate tumor histology,
grade
394,395
and ultimately gene and protein expression
with prognostic implications. The development of more
sophisticated modeling of patient demographic features
as recorded in the electronic medical record, such as
age, gender, race, body mass index, comorbidities,
exposure to tobacco, and other risk factors are being
studied to contextualize and individualize management
options. Finally, tumor markers detected in biopsy,
blood, or urine should be studied to improve prognostic
models for RCC. Efforts based on gene and protein
expression have identified multiple promising markers
that may one day distinguish between subtypes of
malignant and benign renal tumors.
396,397
Recent work
through The Cancer Genome Atlas (TCGA)
398,399
to
identify genomic markers for clear cell RCC
400
, papillary
RCC
401
, and chromophobe RCC
402
holds great clinical
potential for more accurate diagnosis, prognostication,
and surveillance of renal masses. The promise of
measuring circulating tumor cells, or liquid tumor
biopsies, for diagnosis and surveillance for recurrence
and response to treatment is likely several years off,
but could substantially transform care models.
403-406
Counseling and Outcomes-Based Research
As data emerge regarding variability in treatments
performed for localized renal cancer, the impact of the
individual physician-patient interaction becomes more
evident. The quality of patient counseling can only be
improved by providing high quality data, particularly
from RCT’s. Given our current state of knowledge,
translation of information from research studies and
guidelines into practical materials for patients is not
straight-forward. The development of decision aids for
informed medical-decision making is ongoing.
407,408
The
appropriate application of data from large registries and
implementation sciences to improve processes and
standardization of care is an important initiative that
must move forward. Increased quality of data, including
improved assessment of tumor biology and prospective
trials of management options, is greatly needed to
facilitate more intelligent patient counseling.
Management
A major limitation of the literature supporting the
current guidelines for management of localized renal
cancer is the relatively low level of evidence.
Prospective comparative trials, ideally randomized,
comparing AS vs. active intervention (TA or excision)
should be prioritized to provide higher quality data
about oncologic and renal functional outcomes and to
assess the treatment-related morbidities or limitations
of each approach. With improved reporting and more
extended follow-up, multi-institutional observational
data will strengthen confidence in recommendations,
but not nearly to the extent that clinical trials can
provide.
Comparison of extirpative treatment modalities should
include prospective evaluation of PN versus RN,
prioritized in patients with a normal contralateral kidney
and no preexisting CKD/albuminuria, with the goal of
assessing the impact of new baseline functional status
on overall survival, cardiovascular health, and
subsequent renal stability on a longitudinal basis.
Ideally, patients with tumors with increased oncologic
potential (cT1b/T2) should be prioritized for such
trials.
259,409,410
Regarding nephron-sparing surgery,
improved data comparing the relative merits and
limitations of standard PN versus tumor enucleation
should be sought, ideally through prospective
evaluation incorporating improved reporting, and
standard assessment of surgical margins.
248
Multiple non-extirpative methods being actively
investigated in the management of renal masses
include stereotactic body radiation therapy (SBRT),
HIFU, microwave ablation (MWA), and laser interstitial
thermal therapy (LITT). These approaches differ in their
mechanisms of action, invasiveness, reported outcomes
and experience. Their use should be approached
systematically and with caution, and they should be
considered investigational at present. SBRT, also
frequently referred to as stereotactic ablative
radiotherapy (SABR), has been reported in a small
number of series. SBRT involves relatively intense
protocols (24 to 40 Gy) over one to five fractions and a
high degree of spatial precision, offering the potential
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

41
to be less invasive than surgical or conventional
ablative approaches.
411
Despite encouraging results, the
current body of evidence is limited due to small patient
numbers, short follow-up and inconsistent methods of
reporting outcomes.
411
Thus, SBRT in the management
of localized renal masses at present remains
investigational and should be primarily considered for
patients who are medically inoperable and are not
candidates for established TA approaches. Investigation
through clinical trials should be prioritized.
Similarly, HIFU remains investigational in the
management of renal masses, although it is currently
used clinically to treat prostate cancer and uterine
fibroids.
412
HIFU relies on the use of a lens or focused
transducer to deliver high-frequency sound waves to
tissue, typically 1 to 5 MHz. HIFU may be administered
in an entirely noninvasive means similar to
extracorporeal lithotripsy, thus minimizing the risk of
tumor seeding, urinary extravasation or
hemorrhage.
413I
nitial clinical investigations have
established the feasibility of transcutaneous HIFU;
however, distinct regions of renal masses are
frequently left untreated resulting in incomplete
ablation.
414-417
Similar to RFA, MWA delivers electromagnetic energy
through flexible probes inserted into a target lesion.
MWA produces target temperatures (>60° C) more
rapidly than RFA, and, thus, appears to have significant
potential as an ablative modality.
418
LITT uses optical
fibers that are inserted directly into the target tissue to
deliver laser light that is converted into thermal energy.
The most common laser type used in LITT is a
neodymium: yttrium-aluminum-garnet (Nd:YAG)
laser.
419
Outcomes of clinical investigations are limited
due to the small number of treated patients and short
follow-up.
420,421
Given the limited number of published
studies involving HIFU, MWA and LITT and lack of long-
term follow-up, appropriate use of these modalities in
the management of small renal masses remains poorly
defined. Larger prospective trials will be necessary to
develop and assess optimal use, risks and morbidity.
Follow-Up After Intervention
The proposed guidelines for follow-up after intervention
for renal cancer attempt to provide a risk-based
approach to surveillance and monitoring. Few high-
quality studies currently exist to help formulate
surveillance regimens, and many of the Panel’s
recommendations are thus based primarily on expert
opinion. Any cancer surveillance regimen is a balancing
act that includes many variables such as the likelihood
of disease recurrence at various sites, temporal
considerations, the potential benefits of therapeutic
interventions and effectiveness of these modalities
based on timing of recurrence detection, improvements
in diagnostic and initial interventions, patient
characteristics, and the burden and cost of monitoring.
As electronic medical records and quality and safety
initiatives intensify, tracking outcomes of all patients
will become increasingly codified and more usable for
research purposes. These data can then also be used
to inform the proper sequencing, timing, duration, and
type of follow-up that improves patient outcomes with
the most parsimonious monitoring.
Future research to make patient follow-up more
efficient and effective could include one or many of
these modalities: develop circulating biomarkers to
supplement currently available imaging, develop novel
functional imaging, conduct clinical trials to compare
currently available imaging modalities, as well as
clinical trials to guide the frequency of imaging/follow-
up, similar to studies done in testicular cancer (MRC
TE08)
422
, colon cancer (GLIDA)
423
, and non-small cell
lung cancer (IFCT-0302).
424
SUMMARY
In conclusion, improving the management of localized
renal tumors will require a concerted effort among
clinicians and allied fields to develop higher quality
evidence and facilitate more precise estimations of
relative risks and benefits of each therapeutic
approach.
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®

42
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
Abbreviations
Acve Surveillance AS
Agency for Healthcare Research and Quality AHRQ
American College of Radiology ACR
American Society of Nephrology ASN
American Urological Associaon AUA
Angiomyolipoma AML
Birt Hogg-Dubé BHD
Chest X-Ray CXR
Chronic Kidney Disease CKD
College of American Pathologists CAP
Computed Tomography CT
End-Stage Renal Disease ESRD
Esmated Glomerular ltraon rate eGFR
Fine Needle Aspiraon FNA
Glomerular Filtraon Rate GFR
Hereditary Leiomyomatosis RCC HLRCC
Hereditary Papillary Renal Carcinoma HPRC
High Risk HR
High-Intensity Focused Ultrasound HIFU
Intermediate Risk IR
Laser Intersal Thermal Therapy LITT
Low Risk LR
Magnec Resonance Imaging MRI
Microwave Ablaon MWA
Paral Nephrectomy PN
Positron Emission Tomography PET
Pracce Guidelines Commiee PGC
Radical Nephrectomy RN
Radiofrequency Ablaon RFA
Randomized Controlled Trials RCT
Renal Cell Carcinoma RCC
Renal Mass Biopsy RMB
Society of Intervenonal Radiology SIR
Society of Urologic Oncology SUO
Stereotacc Body Radiaon Therapy SBRT
Thermal Ablaon TA
Ultrasound US
Very High Risk VHR
von Hippel-Lindau VHL

43
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
References
1. Stewart SB, Thompson RH, Psutka SP et al: Evaluaon of the naonal comprehensive cancer network and american uro-
logical associaon renal cell carcinoma surveillance guidelines. J Clin Oncol 2014; 32: 4059.
2. Higgins JDA: Assessing quality of included studies in Cochrane Reviews. The Cochrane Collaboraon Methods Groups
Newsleer 2007; 11.
3. Whing PF, Rutjes AW, Westwood ME et al: QUADAS-2: A revised tool for the quality assessment of diagnosc accuracy
studies. Ann Intern Med 2011; 155: 529.
4. Sterne JA, Hernan MA, Reeves BC, et al: ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interven-
ons. BMJ 2016; 355: i4919.
5. Faraday M, Hubbard H, Kosiak B et al: Staying at the cung edge: a review and analysis of evidence reporng and grad-
ing; the recommendaons of the American Urological Associaon. BJU Int 2009; 104: 294.
6. Hsu C and Sandford BA: The Delphi technique: making sense of consensus. Praccal Assessment, Research & Evaluaon
2007; 12: 1.
7. Kukov A, Fosse LK, Ramchandani P et al: Incidence of benign pathologic ndings at paral nephrectomy for solitary
renal mass presumed to be renal cell carcinoma on preoperave imaging. Urology 2006; 68: 737.
8. Thompson RH, Hill JR, Babayev Y et al: Metastac renal cell carcinoma risk according to tumor size. J Urol 2009; 182: 41.
9. Lane BR, Babineau D, Kaan MW et al: A preoperave prognosc nomogram for solid enhancing renal tumors 7 cm or
less amenable to paral nephrectomy. J Urol 2007; 178: 429.
10. Johnson DC, Vukina J, Smith AB et al: Preoperavely misclassied, surgically removed benign renal masses: a systemac
review of surgical series and United States populaon level burden esmate. J Urol 2015; 193: 30.
11. Naonal Cancer Instute SEER: Stat Fact Sheets: Kidney and Renal Pelvis Cancer. Cancer Stascs 2016; hp://
seer.cancer.gov/staacts/html/kidrp.html.
12. Siegel RL, Miller KD and Jemal A: Cancer stascs, 2020. CA Cancer J Clin 2020; 70: 7.
13. Mathew A, Devesa SS, Fraumeni JF et al: Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev 2002;
11: 171.
14. Nguyen MM, Gill IS, Ellison LM: The evolving presentaon of renal carcinoma in the United States: trends from the Sur-
veillance, Epidemiology, and End Results program. J Urol 2006; 176: 2397.
15. Kane CJ, Mallin K, Ritchey J et al: Renal cell cancer stage migraon: analysis of the Naonal Cancer Data Base. Cancer
2008; 113: 78.
16. Smaldone MC, Egleston B, Hollingsworth JM et al: Understanding treatment disconnect and mortality trends in renal cell
carcinoma using tumor registry data. Med Care 2016; Epub ahead of print.
17. Hollingsworth JM, Miller DC, Daignault S et al: Rising incidence of small renal masses: a need to reassess treatment
eect. JNCI 2006; 98: 1331.
18. The American Cancer Society medical and editorial content team: What Are the Risk Factors for Kidney Cancer? Ameri-
can Cancer Society 2016; hps://www.cancer.org/cancer/kidney-cancer/causes-risks-prevenon/risk-
factors.html#wrien_by.
19. Campbell SC, Lane BR: Campbell-Walsh Urology, Malignant Renal Tumors. 11th ed: Elsevier 2016; 1314.
20. Ferlay J, Soerjomataram I, Dikshit R et al: Cancer incidence and mortality worldwide: sources, methods and major
paerns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359.
21. Znaor A, Lortet-Tieulent J, Laversanne M et al: Bray F. Internaonal variaons and trends in renal cell carcinoma inci-
dence and mortality. Eur Urol 2015; 67: 519.
22. Hunt JD, van der Hel OL, McMillan GP et al: Renal cell carcinoma in relaon to cigaree smoking: meta-analysis of 24
studies. Int J Cancer 2005; 114: 101.
23. Lipworth L, Tarone RE, McLaughlin JK: The epidemiology of renal cell carcinoma. J Urol 2006; 176: 2353.
24. Renehan AG, Tyson M, Egger M et al: Body-mass index and incidence of cancer: a systemac review and meta-analysis
of prospecve observaonal studies. Lancet. 2008; 371: 569.
25. Bjorge T, Tretli S, Engeland A: Relaon of height and body mass index to renal cell carcinoma in two million Norwegian
men and women. Am J Epidemiol 2004; 160: 1168.
26. Hakimi AA, Furberg H, Zabor EC et al: An epidemiologic and genomic invesgaon into the obesity paradox in renal cell
carcinoma. J Natl Cancer Inst 2013; 105: 1862.
27. Yang L, Drake BF, Colditz GA et al: Obesity and other cancers. JCO 2016; 34: 4231.

44
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
28. Shapiro JA, Williams MA, Weiss NS et al: Hypertension, anhypertensive medicaon use, and risk of renal cell carcino-
ma. Am J Epidemiol 1999; 149: 521.
29. Weikert S, Boeing H, Pischon T et al: Blood pressure and risk of renal cell carcinoma in the European prospecve inves-
gaon into cancer and nutrion. Am J Epidemiol 2008; 167: 438.
30. Stewart JH, Buccian G, Agodoa L et al: Cancers of the kidney and urinary tract in paents on dialysis for end-stage renal
disease: analysis of data from the United States, Europe, and Australia and New Zealand. JASN 2003; 14: 197.
31. Guha N, Loomis D, Grosse Y et al: Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated sol-
vents, and their metabolites. The Lancet Oncology 2012; 13: 1192.
32. Rashidkhani B, Akesson A, Lindblad P et al: Alcohol consumpon and risk of renal cell carcinoma: a prospecve study of
Swedish women. Int J Cancer 2005; 117: 848.
33. Greving JP, Lee JE, Wolk A et al: Alcoholic beverages and risk of renal cell cancer. Br J Cancer 2007; 97: 429.
34. Lee JE, Mannisto S, Spiegelman D et al: Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: a pooled
analysis of 13 prospecve studies. Cancer Epidemiol Biomarkers Prev 2009; 18: 1730.
35. Rashidkhani B, Lindblad P, Wolk A: Fruits, vegetables and risk of renal cell carcinoma: a prospecve study of Swedish
women. Int J Cancer 2005; 113: 451.
36. Wolk A, Larsson SC, Johansson JE et al: Long-term fay sh consumpon and renal cell carcinoma incidence in women.
Jama 2006; 296: 1371.
37. McCredie M, Pommer W, McLaughlin JK et al: Internaonal renal-cell cancer study. II. Analgesics. Int J Cancer 1995; 60:
345.
38. Linehan WM, Rickes CJ: The metabolic basis of kidney cancer. Semin Cancer Biol 2013; 23: 46.
39. Duey BG, Choyke PL, Glenn G et al: The relaonship between renal tumor size and metastases in paents with von Hip-
pel-Lindau disease. J Urol 2004; 172: 63.
40. Srigley JR, Delahunt B, Eble JN et al: The Internaonal Society of Urological Pathology (ISUP) Vancouver Classicaon of
renal neoplasia. Am J Surg Pathol 2013; 37: 1469.
41. Moch H, Humphrey PA, Ulbright TM et al: WHO classicaon of tumours of the urinary system and male genital organs.
4 ed: Lyon IARC; 2016.
42. Jayson M, Sanders H: Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998; 51: 203.
43. Luciani LG, Cestari R, Tallarigo C: Incidental renal cell carcinoma-age and stage characterizaon and clinical implicaons:
study of 1092 paents (1982-1997). Urology 2000; 56: 58.
44. Sufrin G, Chasan S, Golio A et al: Paraneoplasc and serologic syndromes of renal adenocarcinoma. Semin Urol 1989; 7:
158.
45. Snyder ME, Bach A, Kaan MW et al: Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in
radiological diameter: inuence of sex. J Urol 2006; 176: 2391.
46. Ball MW, Gorin MA, Bhayani SB et al: Preoperave predictors of malignancy and unfavorable pathology for clinical T1a
tumors treated with paral nephrectomy: A mul-instuonal analysis. Urol Oncol 2015; 33: 112.
47. Davenport MS, Perazella MA, Yee J et al: Use of intravenous iodinated contrast media in paents with kidney disease:
Consensus statements from the american college of radiology and the naonal kidney foundaon. Radiology 2020; 294:
660.
48. Weinreb JC, Rodby RA, Yee J et al: Use of intravenous gadolinium-based contrast media in paents with kidney disease:
Consensus statements from the American College of Radiology and the naonal kidney foundaon. Radiology 2021; 298:
28.
49. Lim DJ, Carter MF: Computerized tomography in the preoperave staging for pulmonary metastases in paents with
renal cell carcinoma. J Urol 1993; 150: 1112.
50. Mano R, Vertosick E, Sankin AI et al: Subcenmeter pulmonary nodules are not associated with disease progression in
paents with renal cell carcinoma. J Urol 2015; 193: 776.
51. Seaman E, Golubo ET, Ross S et al: Associaon of radionuclide bone scan and serum alkaline phosphatase in paents
with metastac renal cell carcinoma. Urology 1996; 48: 692.
52. Marshall ME, Pearson T, Simpson W et al: Low incidence of asymptomac brain metastases in paents with renal cell
carcinoma. Urology 1990; 36: 300.
53. Koga S, Tsuda S, Nishikido M et al: The diagnosc value of bone scan in paents with renal cell carcinoma. J Urol 2001;
166: 2126.

45
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
54. Amin MB, Greene FL, Edge SB et al: The eighth edion ajcc cancer staging manual: Connuing to build a bridge from a
populaon-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017; 67: 93.
55. Fuhrman SA, Lasky LC, Limas C: Prognosc signicance of morphologic parameters in renal cell carcinoma. Am J Surg
Pathol 1982; 6: 655.
56. Delahunt B, Cheville JC, Margnoni G et al: The Internaonal Society of Urological Pathology (ISUP) grading system for
renal cell carcinoma and other prognosc parameters. Am J Surg Pathol 2013; 37: 1490.
57. Bretheau D, Lechevallier E, de Fromont M et al: Prognosc value of nuclear grade of renal cell carcinoma. Cancer 1995;
76: 2543.
58. Keegan KA, Schupp CW, Chamie K et al: Histopathology of surgically treated renal cell carcinoma: survival dierences by
subtype and stage. J Urol 2012; 188: 391.
59. Zisman A, Pantuck AJ, Dorey F et al: Improved prognoscaon of renal cell carcinoma using an integrated staging sys-
tem. J Clin Oncol 2001; 19: 1649.
60. Patard JJ, Kim HL, Lam JS et al: Use of the University of California Los Angeles integrated staging system to predict surviv-
al in renal cell carcinoma: an internaonal mulcenter study. J Clin Oncol 2004; 22: 3316.
61. Frank I, Blute ML, Cheville JC et al: An outcome predicon model for paents with clear cell renal cell carcinoma treated
with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002; 168: 2395.
62. Leibovich BC, Blute ML, Cheville JC et al: Predicon of progression aer radical nephrectomy for paents with clear cell
renal cell carcinoma: a stracaon tool for prospecve clinical trials. Cancer 2003; 97: 1663.
63. Zigeuner R, Huerer G, Chromecki T et al: External validaon of the Mayo Clinic stage, size, grade, and necrosis (SSIGN)
score for clear-cell renal cell carcinoma in a single European centre applying roune pathology. Eur Urol 2010; 57: 102.
64. Karakiewicz PI, Suardi N, Capitanio U et al: A preoperave prognosc model for paents treated with nephrectomy for
renal cell carcinoma. Eur Urol 2009; 55: 287.
65. Pierorazio PM, Johnson MH, Patel HD et al: Management of renal masses and localized renal cancer. AHRQ Publicaon
16-EHC001-EF, 2016 #167.
66. Patel HD, Iyoha E, Pierorazio PM et al: A Systemac Review of Research Gaps in the Evaluaon and Management of Lo-
calized Renal Masses. Urology 2016; 98: 14.
67. Morrissey JJ, Mobley J, Figenshau RS et al: Urine aquaporin 1 and perilipin 2 dierenate renal carcinomas from other
imaged renal masses and bladder and prostate cancer. Mayo Clin Proc 2015; 90: 35.
68. Song JB, Morrissey JJ, Mobley JM et al: Urinary aquaporin 1 and perilipin 2: Can these novel markers accurately charac-
terize small renal masses and help guide paent management? Int J Urol 2019; 26: 260.
69. Kaluz S, Kaluzova M, Liao SY et al: Transcriponal control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one
transcripon factor (HIF-1) show? Biochim Biophys Acta 2009; 1795: 162.
70. Sllebroer AB, Mulders PF, Boerman OC et al: Carbonic anhydrase IX in renal cell carcinoma: implicaons for prognosis,
diagnosis, and therapy. Eur Urol 2010; 58: 75.
71. Muselaers S, Mulders P, Oosterwijk E et al: Molecular imaging and carbonic anhydrase IX-targeted radioimmunotherapy
in clear cell renal cell carcinoma. Immunotherapy 2013; 5: 489.
72. Divgi CR, Pandit-Taskar N, Jungbluth AA et al: Preoperave characterizaon of clear-cell renal carcinoma using iodine-
124-labelled anbody chimeric G250 (124I-cG250) and PET in paents with renal masses: a phase I trial. Lancet Oncol
2007; 8: 304.
73. Divgi CR, Uzzo RG, Gatsonis C et al: Positron emission tomography/computed tomography idencaon of clear cell re-
nal cell carcinoma: results from the REDECT trial. J Clin Oncol 2013; 31: 187.
74. Gorin MA, Rowe SP, Baras AS et al: Prospecve evaluaon of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal on-
cocytomas and hybrid oncocyc/chromophobe tumors. Eur Urol 2016; 69: 413.
75. Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J
Urol 2008; 179: 1227.
76. Chawla SN, Crispen PL, Hanlon AL et al: The natural history of observed enhancing renal masses: meta-analysis and re-
view of the world literature. J Urol 2006; 175: 425.
77. Smaldone MC, Kukov A, Egleston BL et al: Small renal masses progressing to metastases under acve surveillance: A
systemac review and pooled analysis. Cancer 2011; 118: 997.
78. Jewe MA, Maar K, Basiuk J et al: Acve surveillance of small renal masses: progression paerns of early stage kidney
cancer. Eur Urol 2011; 60: 39.

46
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
79. Mason RJ, Abdolell M, Troer G et al: Growth kinecs of renal masses: analysis of a prospecve cohort of paents un-
dergoing acve surveillance. Eur Urol 2011; 59: 863.
80. Pierorazio PM, Johnson MH, Ball MW et al: Five-year analysis of a mul-instuonal prospecve clinical trial of delayed
intervenon and surveillance for small renal masses: The DISSRM Registry. Eur Urol 2015; 68: 408.
81. Patel SG, Penson DF, Pabla B et al: Naonal trends in the use of paral nephrectomy: a rising de that has not lied all
boats. J Urol 2012; 187: 816.
82. Bjurlin MA, Walter D, Taksler GB et al: Naonal trends in the ulizaon of paral nephrectomy before and aer the es-
tablishment of AUA guidelines for the management of renal masses. Urology 2013; 82: 1283.
83. Ghani KR, Sukumar S, Sammon JD et al: Pracce paerns and outcomes of open and minimally invasive paral nephrec-
tomy since the introducon of roboc paral nephrectomy: results from the naonwide inpaent sample. J Urol 2014;
191: 907.
84. Kunkle DA, Uzzo RG: Cryoablaon or radiofrequency ablaon of the small renal mass : a meta-analysis. Cancer 2008;
113: 2671.
85. El Dib R, Touma NJ, Kapoor A: Cryoablaon vs radiofrequency ablaon for the treatment of renal cell carcinoma: a meta-
analysis of case series studies. BJU Int 2012; 110: 510.
86. Pirasteh A, Snyder L, Boncher N et al: Cryoablaon vs. radiofrequency ablaon for small renal masses. Acad Radiol 2011;
18: 97.
87. Kattan MW, Reuter V, Motzer RJ et al: A postoperative prognostic nomogram for renal cell carcinoma. J Urol 2001; 166: 63.
88. Sorbellini M, Kaan MW, Snyder ME et al: A postoperave prognosc nomogram predicng recurrence for paents with
convenonal clear cell renal cell carcinoma. J Urol 2005; 173: 48.
89. Zisman A, Pantuck AJ, Wieder J et al: Risk group assessment and clinical outcome algorithm to predict the natural history
of paents with surgically resected renal cell carcinoma. J Clin Oncol 2002; 20: 4559.
90. Karakiewicz PI, Brigan A, Chun FK et al: Mul-instuonal validaon of a new renal cancer-specic survival nomogram.
J Clin Oncol 2007; 25: 1316.
91. Cindolo L, de la Taille A, Messina G et al: A preoperave clinical prognosc model for non-metastac renal cell carcino-
ma. BJU Int 2003; 92: 901.
92. Cindolo L, de la Taille A, Messina G et al: A preoperave clinical prognosc model for non-metastac renal cell carcino-
ma. BJU Int 2003; 92: 901.
93. Yaycioglu O, Roberts WW, Chan T et al: Prognosc assessment of nonmetastac renal cell carcinoma: A clinically based
model. Urology 2001; 58: 141.
94. Correa AF, Jegede O, Haas NB et al: Predicng renal cancer recurrence: Dening limitaons of exisng prognosc models
with prospecve trial-based validaon. J Clin Oncol 2019; 37: 2062.
95. Ljungberg B, Albiges L, Abu-Ghanem Y et al: European associaon of urology guidelines on renal cell carcinoma: The
2019 update. Eur Urol 2019; 75: 799.
96. Blacher E, Johnson DE and Haynie TP: Value of roune radionuclide bone scans in renal cell carcinoma. Urology 1985;
26: 432.
97. Lindner A, Goldman DG and deKernion JB: Cost eecve analysis of prenephrectomy radioisotope scans in renal cell car-
cinoma. Urology 1983; 22: 127.
98. Benson MA, Haaga JR and Resnick MI: Staging renal carcinoma. What is sucient? Arch Surg 1989; 124: 71.
99. Grünwald V, Eberhardt B, Bex A et al: An interdisciplinary consensus on the management of bone metastases from renal
cell carcinoma. Nat Rev Urol 2018; 15: 511.
100. Davenport MS, Hu EM, Smith AD et al: Reporng standards for the imaging-based diagnosis of renal masses on CT and
MRI: a naonal survey of academic abdominal radiologists and urologists. Abdom Radiol 2017: 42; 1229.
101. Berland LL, Silverman SG, Gore RM et al: Managing incidental ndings on abdominal CT: white paper of the ACR inci-
dental ndings commiee. J Am Coll Radiol 2010; 7: 754.
102. Richmond L, Atri M, Sherman C et al: Renal cell carcinoma containing macroscopic fat on CT mimics angiomyolipoma due
to bone metapolasia without macroscopic calcicaon. Br J Radiol 2010; 83: e179.
103. Rofsky NM, Weinreb JC, Bosniak MA et al: Renal lesion characterizaon with gadolinium-enhanced MR imaging: ecacy
and safety in paents with renal insuciency. Radiology 1991; 180: 85.
104. Schoots IG, Zaccai K, Hunink MG et al: Bosniak classicaon for complex renal cysts reevaluated: A systemac review. J
Urol 2017; 198: 12.

47
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
105. Silverman SG, Pedrosa I, Ellis JH et al: Bosniak classicaon of cysc renal masses, version 2019: An update proposal and
needs assessment. Radiology 2019; 292: 475.
106. Nicolau C, Buñesch L, Paño B et al: Prospecve evaluaon of CT indeterminate renal masses using US and contrast-
enhanced ultrasound. Abdom Imaging 2015; 40: 542.
107. Atri M, Tabatabaeifar L, Jang H-J et al: Accuracy of contrast-enhanced US for dierenang benign from malignant solid
small renal masses. Radiology 2015; 276: 900.
108. Kopp RP, Aganovic L, Palazzi KL et al: Dierenaon of clear from non-clear cell renal cell carcinoma using CT washout
formula. Can J Urol 2013; 20: 6790.
109. Young JR, Margolis D, Sauk S et al: Clear cell renal cell carcinoma: discriminaon from other renal cell carcinoma sub-
types and oncocytoma at mulphasic muldetector CT. Radiology 2013; 267: 444.
110. Kukov A, Uzzo RG: The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantang renal tu-
mor size, locaon and depth. J Urol 2009; 182: 844.
111. Ficarra V, Novara G, Secco S et al: Preoperave aspects and dimensions used for an anatomical (PADUA) classicaon of
renal tumours in paents who are candidates for nephron-sparing surgery. Eur Urol 2009; 56: 786.
112. Simmons MN, Ching CB, Samplaski MK et al: Kidney tumor locaon measurement using the C index method. J Urol 2010;
183: 1708.
113. Tobert CM, Kahnoski RJ, Thompson DE et al: RENAL nephrometry score predicts surgery type independent of individual
surgeon’s use of nephron-sparing surgery. Urology 2012; 80: 157.
114. Stroup SP, Palazzi K, Kopp RP et al: RENAL nephrometry score is associated with operave approach for paral nephrec-
tomy and urine leak. Urology 2012; 80: 151.
115. Bruner B, Breau RH, Lohse CM et al: Renal nephrometry score is associated with urine leak aer paral nephrectomy.
BJU Int 2011; 108: 67.
116. Kukov A, Smaldone M, Egleston BL et al: Anatomic features of enhancing renal masses predict malignant and high-
grade pathology: a preoperave nomogram using the RENAL Nephrometry score. Eur Uro 2011; 60: 241.
117. Mehrazin R, Palazzi KL, Kopp RP et al: Impact of tumour morphology on renal funcon decline aer paral nephrectomy.
BJU Int 2013; 111: E374.
118. Levey AS, Eckardt KU, Tsukamoto Y et al: Denion and classicaon of chronic kidney disease: a posion statement
from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089.
119. Hallan SI, Ritz E, Lydersen S et al: Combining GFR and albuminuria to classify CKD improves predicon of ESRD. J Am Soc
Nephrol 2009; 20: 1069.
120. Levey AS, Bosch JP, Lewis JB et al: A more accurate method to esmate glomerular ltraon rate from serum creanine:
a new predicon equaon. Modicaon of diet in renal disease study group. Ann Intern Med 1999; 130: 461.
121. Levey AS, Stevens LA, Schmid CH et al: A new equaon to esmate glomerular ltraon rate. Ann Intern Med 2009; 150:
604.
122. Barocas DA, Boorjian SA, Alvarez RD et al: Microhematuria: Aua/sufu guideline. J Urol 2020; 204: 778.
123. Pinkham CA, Krause KJ: Liver funcon tests and mortality in a cohort of life insurance applicants. J Insur Med 2009; 41:
170.
124. Guðmundsson E, Hellborg H, Lundstam S et al: Metastac potenal in renal cell carcinomas ≤7 cm: Swedish Kidney Can-
cer Quality Register data. Eur Urol 2011; 60: 975.
125. Umbreit EC, Shimko MS, Childs MA et al: Metastac potenal of a renal mass according to original tumour size at
presentaon. BJU Int 2012; 109: 190.
126. Barlow LJ, Korets R, Laudano M et al: Predicng renal funconal outcomes aer surgery for renal corcal tumours: a
mulfactorial analysis. BJU Int 2010; 106: 489.
127. Kim SH, Lee SE, Hong SK et al: Incidence and risk factors of chronic kidney disease in Korean paents with t1a renal cell
carcinoma before and aer radical or paral nephrectomy. Jpn J Clin Oncol 2013; 43: 1243.
128. Jeon HG, Jeong IG, Lee JW et al: Prognosc factors for chronic kidney disease aer curave surgery in paents with small
renal tumors. Urology 2009; 74: 1064.
129. Huang WC, Levey AS, Serio AM et al: Chronic kidney disease aer nephrectomy in paents with renal corcal tumours: a
retrospecve cohort study. Lancet Oncol 2006; 7: 735.
130. Choi YS, Park YH, Kim YJ et al: Predicve factors for the development of chronic renal insuciency aer renal surgery: a
mulcenter study. Int Urol Nephrol 2014; 46: 681.

48
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
131. Takagi T, Kondo T, Iizuka J et al: Postoperave renal funcon aer paral nephrectomy for renal cell carcinoma in pa-
ents with pre-exisng chronic kidney disease: a comparison with radical nephrectomy. Int J Urol 2011; 18: 472.
132. Clark MA, Shikanov S, Raman JD et al: Chronic kidney disease before and aer paral nephrectomy. J Urol 2011; 185: 43.
133. Hung PH, Tsai HB, Hung KY et al: Increased risk of end-stage renal disease in paents with renal cell carcinoma: a 12-year
naonwide follow-up study. Medicine (Balmore) 2014; 93: e52.
134. Go AS, Chertow GM, Fan D et al: Chronic kidney disease and the risks of death, cardiovascular events, and hospitaliza-
on. N Engl J Med 2004; 351: 1296.
135. Gerstein HC, Mann JF, Yi Q et al: Albuminuria and risk of cardiovascular events, death, and heart failure in diabec and
nondiabec individuals. Jama 2001; 286: 421.
136. Tourojman M, Kirmiz S, Boelkins B et al: Impact of Reduced Glomerular Filtraon Rate and Proteinuria on Overall Surviv-
al of Paents with Renal Cancer. J Urol 2016; 195: 588.
137. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Pracce Guideline for the
Evaluaon and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1.
138. Bachrach L, Negron E, Liu JS et al: Preoperave nuclear renal scan underesmates renal funcon aer radical nephrecto-
my. Urology 2014; 84: 1402.
139. Sankin A, Sfakianos JP, Schi J et al: Assessing renal funcon aer paral nephrectomy using renal nuclear scingraphy
and esmated glomerular ltraon rate. Urology 2012; 80: 343.
140. Patel SR, Abel EJ, Hedican SP et al: Ablaon of small renal masses: pracce paerns at academic instuons in the Unit-
ed States. J Endourol 2013; 27: 158.
141. Menhadji AD, Nguyen V, Okhunov Z et al: Technique for oce-based, ultrasonography-guided percutaneous biopsy of
renal corcal neoplasms using a novel transducer for facilitated ultrasound targeng. BJU Int 2016; 117: 948.
142. Bearrick EN, Packiam V, Bhindi B et al: Creaon of a primary tumor ssue expression biomarker-augmented prognosc
model for paents with metastac renal cell carcinoma. Urol Oncol 2021; 39: 135.e1.
143. Tosoian JJ, Feldman AS, Abbo MR et al: Biopsy cell cycle proliferaon score predicts adverse surgical pathology in local-
ized renal cell carcinoma. Eur Urol 2020; 78: 657.
144. Bijol V, Mendez GP, Hurwitz S et al: Evaluaon of the nonneoplasc pathology in tumor nephrectomy specimens: pre-
dicng the risk of progressive renal failure. Am J Surg Pathol 2006; 30: 575.
145. Salvatore SP, Cha EK, Roso JS et al: Nonneoplasc renal corcal scarring at tumor nephrectomy predicts decline in kid-
ney funcon. Arch Pathol Lab Med 2013; 137: 531.
146. Henriksen KJ, Meehan SM, Chang A: Non-neoplasc renal diseases are oen unrecognized in adult tumor nephrectomy
specimens: a review of 246 cases. Am J Surg Pathol 2007; 31: 1703.
147. Rini BI, Plimack ER, Takagi T et al: A Phase II Study of Pazopanib in Paents with Localized Renal Cell Carcinoma to Op-
mize Preservaon of Renal Parenchyma. J Urol 2015; 194: 297.
148. Karam JA, Devine CE, Urbauer DL et al: Phase 2 trial of neoadjuvant axinib in paents with locally advanced nonmet-
astac clear cell renal cell carcinoma. Eur Urol 2014; 66: 874.
149. Bhindi B, Thompson RH, Lohse CM et al: The probability of aggressive versus indolent histology based on renal tumor
size: Implicaons for surveillance and treatment. Eur Urol 2018; 74: 489.
150. Frank I, Blute ML, Cheville JC et al: Solid renal tumors: an analysis of pathological features related to tumor size. J Urol
2003; 170: 2217.
151. Pierorazio PM, Hyams ES, Tsai S et al: Mulphasic enhancement paerns of small renal masses (</=4 cm) on preopera-
ve computed tomography: ulity for disnguishing subtypes of renal cell carcinoma, angiomyolipoma, and oncocyto-
ma. Urology 2013; 81: 1265.
152. Chandrasekar T, Ahmad AE, Fadaak K et al: Natural history of complex renal cysts: Clinical evidence supporng acve
surveillance. J Urol 2018; 199: 633.
153. Kashan M, Ghanaat M, Hötker AM et al: Cysc renal cell carcinoma: A report on outcomes of surgery and acve surveil-
lance in paents retrospecvely idened on pretreatment imaging. J Urol 2018; 200: 275.
154. Westerman ME, Cheville JC, Lohse CM et al: Long-term outcomes of paents with low grade cysc renal epithelial neo-
plasms. Urology 2019; 133: 145.
155. Kukov A, Egleston BL, Canter D et al: Compeng risks of death in paents with localized renal cell carcinoma: a comor-
bidity based model. J Urol 2012; 188: 2077.

49
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
156. Patel HD, Kates M, Pierorazio PM et al: Balancing cardiovascular (CV) and cancer death among paents with small renal
masses: modicaon by CV risk. BJU Int 2015; 115: 58.
157. Patel HD, Kates M, Pierorazio PM et al: Comorbidies and Causes of Death in the Management of Localized T1a Kidney
Cancer. Int J Urol 2014;arcle in press.
158. Bianchi M, Becker A, Abdollah F et al: Rates of open versus laparoscopic and paral versus radical nephrectomy for T1a
renal cell carcinoma: a populaon-based evaluaon. Int J Urol 2013; 20: 1064.
159. McIntosh AG, Ristau BT, Ruth K et al: Acve surveillance for localized renal masses: Tumor growth, delayed intervenon
rates, and >5-yr clinical outcomes. Eur Urol 2018; 74: 157.
160. Somehin AE, Patel HD, Alam R et al: Selecng paents with small renal masses for acve surveillance: A domain based
score from a prospecve cohort study. J Urol 2019; 201: 886.
161. Mir MC, Capitanio U, Bertolo R et al: Role of acve surveillance for localized small renal masses. Eur Urol Oncol 2018; 1:
177.
162. Chow W-H, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010; 7: 245.
163. Wu J, Suk-Ouichai C, Dong W et al: Analysis of survival for paents with chronic kidney disease primarily related to renal
cancer surgery. BJU Int 2018; 121: 93.
164. Lane BR, Demirjian S, Derweesh IH et al: Survival and funconal stability in chronic kidney disease due to surgical remov-
al of nephrons: Importance of the new baseline glomerular ltraon rate. Eur Urol 2015; 68: 996.
165. Lane BR, Campbell SC, Demirjian S et al: Surgically induced chronic kidney disease may be associated with a lower risk of
progression and mortality than medical chronic kidney disease. J Urol 2013; 189: 1649.
166. Li L, Lau WL, Rhee CM et al: Risk of chronic kidney disease aer cancer nephrectomy. Nat Rev Nephrol 2014; 10: 135.
167. Malcolm JB, Bagrodia A, Derweesh IH et al: Comparison of rates and risk factors for developing chronic renal insucien-
cy, proteinuria and metabolic acidosis aer radical or paral nephrectomy. BJU Int 2009; 104: 476.
168. Sles KP, Moa MJ, Agodoa LY et al: Renal cell carcinoma as a cause of end-stage renal disease in the United States:
Paent characteriscs and survival. Kidney Int 2003; 64: 247.
169. Jeon HG, Choo SH, Sung HH et al: Small tumour size is associated with new-onset chronic kidney disease aer radical
nephrectomy in paents with renal cell carcinoma. Eur J Cancer 2014; 50: 64.
170. Cho A, Lee JE, Kwon G-Y et al: Post-operave acute kidney injury in paents with renal cell carcinoma is a potent risk
factor for new-onset chronic kidney disease aer radical nephrectomy. Nephrol Dial Transpl 2011; 26: 3496.
171. Thadhani R, Pascual M, Bonventre JV: Acute Renal Failure. N Engl J Med 1996; 334: 1448.
172. Jacobson HR: Chronic renal failure: pathophysiology. Lancet 1991; 338: 419.
173. Nagata M, Kriz W: Glomerular damage aer uninephrectomy in young rats. II. Mechanical stress on podocytes as a path-
way to sclerosis. Kidney Int 1992; 42: 148.
174. Romagnani P, Remuzzi G, Glassock R et al: Chronic kidney disease. Nat Rev Dis Primers 2017; 3: 17088.
175. Webster AC, Nagler EV, Morton RL et al: Chronic kidney disease. Lancet 2017; 389: 1238.
176. Huang WC, Donin NM, Levey AS et al: Chronic kidney disease and kidney cancer surgery: New perspecves. J Urol 2020;
203: 475.
177. Linehan WM: Genec basis of kidney cancer: Role of genomics for the development of disease-based therapeucs. Ge-
nome Res 2012; 22: 2089.
178. Menko FH, Maher ER, Schmidt LS et al: Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, sur-
veillance and treatment. Fam Cancer 2014; 13: 637.
179. Gudbjartsson T, Jónasdór TJ, Thoroddsen A et al: A populaon-based familial aggregaon analysis indicates genec
contribuon in a majority of renal cell carcinomas. Int J Cancer 2002; 100: 476.
180. Hampel H, Benne RL, Buchanan A et al: A pracce guideline from the american college of medical genecs and ge-
nomics and the naonal society of genec counselors: Referral indicaons for cancer predisposion assessment. Genet
Med 2015; 17: 70.
181. Motzer RJ, Jonasch E, Boyle S et al: Nccn guidelines insights: Kidney cancer, version 1.2021. J Natl Compr Canc Netw
2020; 18: 1160.
182. Shuch B, Vourgan S, Rickes CJ et al: Dening early-onset kidney cancer: implicaons for germline and somac muta-
on tesng and clinical management. J Clin Oncol 2014; 32: 431.
183. Marconi L, Dabestani S, Lam TB et al: Systemac review and meta-analysis of diagnosc accuracy of percutaneous renal
tumor biopsy. Euro Urology 2016; 69: 660.
184. Kukov A, Smaldone MC, Uzzo RG et al: Renal mass biopsy: always, somemes, or never? Euro Urology 2016; 70: 403.

50
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
185. Ingels A, Barret E, Sanchez-Salas R et al: Percutaneous renal biopsies for small renal masses: Complex tumors on
nephrometry should be the rst targets. Clinical Genitourinary Cancer 2016; 14: e457.
186. Hoare D, Evans H, Richards H et al: Evaluang the role for renal biopsy in t1 and t2 renal masses: A single-centre study.
Canadian Urological Associaon Journal 2018; 12: E226.
187. Garbens A, Wallis CJD, Klaassen Z et al: Comprehensive assessment of the morbidity of renal mass biopsy: A populaon-
based assessment of biopsy-related complicaons. Can Urol Assoc J 2020;
188. Wang X, Lv Y, Xu Z et al: Accuracy and safety of ultrasound-guided percutaneous needle core biopsy of renal masses: A
single center experience in china. Medicine 2018; 97: e0178.
189. Alle N, Tan N, Huss J et al: Percutaneous image-guided core biopsy of solid renal masses: Analysis of safety, ecacy,
pathologic interpretaon, and clinical signicance. Abdominal Radiology 2018; 43: 1813.
190. Tong W, Lin X, Xu Y et al: The role of percutaneous ne needle aspiraon biopsy in the management of small renal mass-
es without chance of nephron-sparing surgery. Int Urol Nephrol 2020; 52: 2223.
191. Prendeville S, Richard PO, Jewe MAS et al: Accuracy of renal tumour biopsy for the diagnosis and subtyping of papillary
renal cell carcinoma: Analysis of paired biopsy and nephrectomy specimens with focus on discordant cases. J Clin Pathol
2019; 72: 363.
192. Ginzburg S, Uzzo R, Al-Saleem T et al: Coexisng hybrid malignancy in a solitary sporadic solid benign renal mass: impli-
caons for treang paents following renal biopsy. J Urol 2014; 191: 296.
193. Patel HD, Druskin SC, Rowe SP et al: Surgical histopathology for suspected oncocytomas on renal mass biopsy: a system-
ac review and meta-analysis. BJU Int 2017; Epub ahead of print.
194. Huang H, Tamboli P, Karam JA et al: Secondary malignancies diagnosed using kidney needle core biopsies: a clinical and
pathological study of 75 cases. Hum Pathol 2016; 52: 55.
195. Adamy A, Von Bodman C, Ghoneim T et al: Solitary, isolated metastac disease to kidney: Memorial Sloan-Keering Can-
cer Center experience. BJU Int 2011; 108: 338.
196. Surabhi VR, Menias C, Prasad SR et al: Neoplasc and non-neoplasc proliferave disorders of the perirenal space: cross
-seconal imaging ndings. Radiographics 2008; 28: 1005.
197. Duveau A, Sayegh J, Beloncl F et al: Pseudotumours: an atypical presentaon of renal sarcoidosis. QJM 2013; 106: 947.
198. Dufour JF, Le Gallou T, Cordier JF et al: Urogenital manifestaons in Wegener granulomatosis: a study of 11 cases and
review of the literature. Medicine (Balmore) 2012; 91: 67.
199. Li JY, Yong TY, Coleman M et al: Bilateral renal inammatory pseudotumour eecvely treated with corcosteroid. Clin
Exp Nephrol 2010; 14: 190.
200. Patel HD, Johnson MH, Pierorazio PM et al: Diagnosc accuracy and risks of biopsy in the diagnosis of a renal mass suspi-
cious for localized renal cell carcinoma: systemac review of the literature. J Urol 2016; 195: 1340.
201. Richard PO, Jewe MA, Bha JR et al: Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur
Urol 2015; 68: 1007.
202. Aguilar Palacios D, Li J, Mahmood F et al: Paral nephrectomy for paents with severe chronic kidney disease-is it worth-
while? J Urol 2020; 204: 434.
203. American Society of Cytopathology. ASC Rapid On-Site Evaluaon (ROSE) Posion Statement. Cytopahtology 2014;
hp://www.cytopathology.org/wp-content/uploads/2013/05/ASC-ROSE-Posion-Final-Commiee-Document.pdf.
204. Pierorazio PM, Johnson MH, Patel HD et al: Management of renal masses and localized renal cancer: systemac review
and meta-analysis. J Urol 2016; 196: 989.
205. Van Poppel H, Da Pozzo L, Albrecht W et al: A prospecve, randomized EORTC intergroup phase 3 study comparing the
oncologic outcome of elecve nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur
Urol 2011; 59: 543.
206. Wiklund F, Tretli S, Choueiri TK et al: Risk of bilateral renal cell cancer. J Clin Oncol 2009; 27: 3737.
207. Kheterpal E and Taneja SS: Paral nephrectomy: Contemporary outcomes, candidate selecon, and surgical approach.
Urol Clin North Am 2012; 39: 199.
208. Touijer K, Jacqmin D, Kavoussi LR et al: The expanding role of paral nephrectomy: A crical analysis of indicaons, re-
sults, and complicaons. Eur Urol 2010; 57: 214.
209. Alam R, Patel HD, Osumah T et al: Comparave eecveness of management opons for paents with small renal mass-
es: A prospecve cohort study. BJU Int 2019; 123: 42.

51
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
210. Park BK, Gong IH, Kang MY et al: Rfa versus roboc paral nephrectomy for t1a renal cell carcinoma: A propensity score-
matched comparison of mid-term outcome. Eur Radiol 2018; 28: 2979.
211. Andrews JR, Atwell T, Schmit G et al: Oncologic outcomes following paral nephrectomy and percutaneous ablaon for
ct1 renal masses. Eur Urol 2019; 76: 244.
212. Talenfeld AD, Gennarelli RL, Elkin EB et al: Percutaneous ablaon versus paral and radical nephrectomy for t1a renal
cancer: A populaon-based analysis. Ann Intern Med 2018; 169: 69.
213. Herring JC, Enquist EG, Cherno A et al: Parenchymal sparing surgery in paents with hereditary renal cell carcinoma: 10
-year experience. J Urol 2001; 165: 777.
214. Campbell SC, Novick AC, Belldegrun A et al: Guideline for management of the clinical T1 renal mass. J Urol 2009; 182:
1271.
215. Uzzo RG, Novick AC: Nephron sparing surgery for renal tumors: indicaons, techniques and outcomes. J Urol 2001; 166:
6.
216. Nguyen CT, Campbell SC, Novick AC: Choice of operaon for clinically localized renal tumor. Urol Clin North Am 2008; 35:
645.
217. Fergany AF, Saad IR, Woo L et al: Open paral nephrectomy for tumor in a solitary kidney: experience with 400 cases. J
Urol 2006; 175: 1630.
218. Ghavamian R, Cheville JC, Lohse CM et al: Renal cell carcinoma in the solitary kidney: an analysis of complicaons and
outcome aer nephron sparing surgery. J Urol 2002; 168: 454.
219. Lane BR, Demirjian S, Derweesh IH et al: Survival and funconal stability in chronic kidney disease due to surgical remov-
al of nephrons: importance of the new baseline glomerular ltraon rate. Eur Urol 2015; 68: 996.
220. Demirjian S, Lane BR, Derweesh IH et al: Chronic kidney disease due to surgical removal of nephrons: relave rates of
progression and survival. J Urol 2014; 192: 1057.
221. Lane BR, Russo P, Uzzo RG et al: Comparison of cold and warm ischemia during paral nephrectomy in 660 solitary kid-
neys reveals predominant role of nonmodiable factors in determining ulmate renal funcon. J Urol 2011; 185: 421.
222. Stevens PE, Levin A: Evaluaon and management of chronic kidney disease: synopsis of the kidney disease: improving
global outcomes 2012 clinical pracce guideline. Ann Intern Med 2013; 158: 825.
223. Scosyrev E, Messing EM, Sylvester R et al: Renal funcon aer nephron-sparing surgery versus radical nephrectomy:
results from EORTC randomized trial 30904. Eur Urol 2014; 65: 372.
224. Antonelli A, Mari A, Longo N et al: Role of clinical and surgical factors for the predicon of immediate, early and late
funconal results, and its relaonship with cardiovascular outcome aer paral nephrectomy: Results from the prospec-
ve mulcenter record 1 project. J Urol 2018; 199: 927.
225. Rosen DC, Kannappan M, Kim Y et al: The impact of obesity in paents undergoing roboc paral nephrectomy. J En-
dourol 2019; 33: 431.
226. Isharwal S, Ye W, Wang A et al: Impact of comorbidies on funconal recovery from paral nephrectomy. J Urol 2018;
199: 1433.
227. Volpe A, Blute ML, Ficarra V et al: Renal ischemia and funcon aer paral nephrectomy: a collaborave review of the
literature. Eur Urol 2015; 68: 61.
228. Mir MC, Ercole C, Takagi T et al: Decline in renal funcon aer paral nephrectomy: eology and prevenon. J Urol
2015; 193: 1889.
229. Thompson RH, Lane BR, Lohse CM et al: Renal funcon aer paral nephrectomy: eect of warm ischemia relave to
quanty and quality of preserved kidney. Urology 2012; 79: 356.
230. Wu J, Suk-Ouichai C, Dong W et al: Vascularized parenchymal mass preserved with paral nephrectomy: Funconal im-
pact and predicve factors. Eur Urol Oncol 2019; 2: 97.
231. Dong W, Wu J, Suk-Ouichai C et al: Ischemia and funconal recovery from paral nephrectomy: Rened perspecves.
Eur Urol Focus 2018; 4: 572.
232. Takagi T, Mir MC, Campbell RA et al: Predictors of precision of excision and reconstrucon in paral nephrectomy. J Urol
2014; 192: 30.
233. Zhang Z, Zhao J, Velet L et al: Funconal recovery from extended warm ischemia associated with paral nephrectomy.
Urology 2016; 87: 106.
234. Zhang Z, Zhao J, Dong W et al: Acute kidney injury aer paral nephrectomy: role of parenchymal mass reducon and
ischemia and impact on subsequent funconal recovery. Eur Urol 2016; 69: 745.

52
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
235. Bravi CA, Larcher A, Capitanio U et al: Perioperave outcomes of open, laparoscopic, and roboc paral nephrectomy: A
prospecve mulcenter observaonal study (the record 2 project). Eur Urol Focus 2019;
236. Satkunasivam R, Tsai S, Syan S et al: Roboc unclamped "minimal-margin" paral nephrectomy: ongoing renement of
the anatomic zero-ischemia concept. Eur Urol 2015; 68: 705.
237. Hung AJ, Cai J, Simmons MN et al: "Trifecta" in paral nephrectomy. J Urol 2013; 189: 36.
238. Ng CK, Gill IS, Pal MB et al: Anatomic renal artery branch microdissecon to facilitate zero-ischemia paral nephrecto-
my. Eur Urol 2012; 61: 67.
239. Simone G, Gill IS, Morie A et al: Indicaons, techniques, outcomes, and limitaons for minimally ischemic and o-
clamp paral nephrectomy: A systemac review of the literature. Eur Urol 2015; 68: 632.
240. Cacciamani GE, Medina LG, Gill TS et al: Impact of renal hilar control on outcomes of roboc paral nephrectomy: Sys-
temac review and cumulave meta-analysis. Eur Urol Focus 2019; 5: 619.
241. Khalifeh A, Kaouk JH, Bhayani S et al: Posive surgical margins in robot-assisted paral nephrectomy: a mul-
instuonal analysis of oncologic outcomes (leave no tumor behind). J Urol 2013; 190: 1674.
242. Shah PH, Moreira DM, Okhunov Z et al: Posive surgical margins increase risk of recurrence aer paral nephrectomy
for high risk renal tumors. J Urol 2016; 196: 327.
243. Walther MM, Thompson N, Linehan W: Enucleaon procedures in paents with mulple hereditary renal tumors. World
J Urol 1995; 13: 248.
244. Minervini A, Tuccio A, Masieri L et al: Endoscopic robot-assisted simple enucleaon (ERASE) for clinical T1 renal masses:
descripon of the technique and early postoperave results. Surg Endosc 2015; 29: 1241.
245. Laryngakis NA, Guzzo TJ: Tumor enucleaon for small renal masses. Curr Opin Urol 2012; 22: 365.
246. Minervini A, Campi R, Lane BR et al: Impact of resecon technique on perioperave outcomes and surgical margins aer
paral nephrectomy for localized renal masses: A prospecve mulcenter study. J Urol 2020; 203: 496.
247. Lu Q, Ji C, Zhao X et al: Histopathologic analysis of tumor bed and peritumoral pseudocapsule aer in vitro tumor enu-
cleaon on radical nephrectomy specimen for clinical t1b renal cell carcinoma. Urol Oncol 2017; 35: 603.e15.
248. Gupta GN, Boris RS, Zhang Z et al: Tumor enucleaon for sporadic localized kidney cancer: opposing views. J Urol 2015;
194: 635.
249. Jacob JM, Williamson SR, Gondim DD et al: Characteriscs of the peritumoral pseudocapsule vary predictably with histo-
logic subtype of T1 renal neoplasms. Urology 2015; 86: 956.
250. Li QL, Guan HW, Zhang QP et al: Opmal margin in nephron-sparing surgery for renal cell carcinoma 4 cm or less. Eur
Urol 2003; 44: 448.
251. Xi W, Wang J, Liu L et al: Evaluaon of tumor pseudocapsule status and its prognosc signicance in renal cell carcino-
ma. J Urol 2018; 199: 915.
252. Minervini A, di Cristofano C, Lapini A et al: Histopathologic analysis of peritumoral pseudocapsule and surgical margin
status aer tumor enucleaon for renal cell carcinoma. Eur Urol 2009; 55: 1410.
253. Bansal RK, Tanguay S, Finelli A et al: Posive surgical margins during paral nephrectomy for renal cell carcinoma: Re-
sults from canadian kidney cancer informaon system (ckcis) collaborave. Can Urol Assoc J 2017; 11: 182.
254. Dash A, Vickers AJ, Schachter LR et al: Comparison of outcomes in elecve paral vs radical nephrectomy for clear cell
renal cell carcinoma of 4-7 cm. BJU Int 2006; 97: 939.
255. Simmons MN, Weight CJ, Gill IS et al: Laparoscopic radical versus paral nephrectomy for tumors > 4 cm: intermediate-
term oncologic and funconal outcomes. Urology 2009; 73: 1077.
256. Crépel M, Jeldres C, Perroe P et al: Nephron-sparing surgery is equally eecve to radical nephrectomy for T1BN0M0
renal cell carcinoma: a populaon-based assessment. Urology 2010; 75: 271.
257. Badalato GM, Kates M, Wisnivesky JP et al: Survival aer paral and radical nephrectomy for the treatment of stage
T1bN0M0 renal cell carcinoma (RCC) in the USA: a propensity scoring approach. BJU Int 2012; 109: 1457.
258. Kim SP, Thompson RH, Boorjian SA et al: Comparave eecveness for survival and renal funcon of paral and radical
nephrectomy for localized renal tumors: a systemac review and meta-analysis. J Urol 2012; 188: 51.
259. Weight CJ, Miller DC, Campbell SC et al: The management of a cT1b renal tumor in the presence of a normal contrala-
teral kidney. J Urol 2013; 189: 1198.
260. Lee HJ, Liss MA, Derweesh IH: Outcomes of paral nephrectomy for clinical T1b and T2 renal tumors. Curr Opin Urol
2014; 24: 448.
261. Tomaszewski JJ, Smaldone MC, Uzzo RG et al: Is radical nephrectomy a legimate therapeuc opon in paents with
renal masses amenable to nephron-sparing surgery?. BJU Int 2015; 115: 357.

53
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
262. Crispen PL, Boorjian SA, Lohse CM et al: Outcomes following paral nephrectomy by tumor size. J Urol 2008; 180: 1912.
263. Simmons MN, Herts B, Campbell SC et al: Image-based approaches to the diagnosis and treatment of renal masses. AUA
Update Series 2007; 26: 381.
264. Ishigami K, Leite LV, Pakalniskis MG et al: Tumor grade of clear cell renal cell carcinoma assessed by contrast-enhanced
computed tomography. Springer plus 2014; 26: 694.
265. Crane A, Suk-Ouichai C, Campbell JA et al: Imprudent ulizaon of paral nephrectomy. Urology 2018; 117: 22.
266. Simhan J, Smaldone MC, Tsai KJ et al: Objecve measures of renal mass anatomic complexity predict rates of major com-
plicaons following paral nephrectomy. Eur Urol 2011; 60: 724.
267. Potretzke AM, Knight BA, Zargar H et al: Urinary stula aer robot-assisted paral nephrectomy: a mulcentre analysis
of 1791 paents. BJU Int 2016; 117: 131.
268. Van Poppel H, Da Pozzao L, Albrect W et al: A prospecve, randomized EORTC intergroup phase 3 study comparing the
oncologic outcome of elecve nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur
Urol 2011; 59: 543.
269. Breau RH, Kapoor A, Nash DM et al: Paral vs. Radical nephrectomy and the risk of all-cause mortality, cardiovascular,
and nephrological outcomes. Can Urol Assoc J 2020; 14: 337.
270. Streja E, Kalantar-Zadeh K, Molnar MZ et al: Radical versus paral nephrectomy, chronic kidney disease progression and
mortality in us veterans. Nephrol Dial Transplant 2018; 33: 95.
271. Kim SP, Campbell SC, Gill I et al: Collaborave review of risk benet trade-os between paral and radical nephrectomy
in the management of anatomically complex renal masses. Eur Urol 2017; 72: 64.
272. Ye Y, Tanaka H, Wang Y et al: Split renal funcon in paents with renal masses: Ulity of parenchymal volume analysis vs
nuclear renal scans. BJU Int 2020; 125: 686.
273. Capitanio U, Becker F, Blute ML et al: Lymph node dissecon in renal cell carcinoma. Eur Urol 2011; 60: 1212.
274. Bekema HJ, MacLennan S, Imamura M et al: Systemac review of adrenalectomy and lymph node dissecon in locally
advanced renal cell carcinoma. Eur Urol 2013; 64: 799.
275. Blom JH, van Poppel H, Maréchal JM et al: Radical nephrectomy with and without lymph-node dissecon: nal results of
European Organizaon for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol 2009;
55: 28.
276. Gershman B, Moreira D, Thompson H et al: Radical nephrectomy with or without lymph node dissecon for nonmet-
astac renal cell carcinoma: a propensity score-based analysis. Eur Urol 2017; 71: 560.
277. Bhindi B, Wallis CJD, Boorjian SA et al: The role of lymph node dissecon in the management of renal cell carcinoma: A
systemac review and meta-analysis. BJU Int 2018; 121: 684.
278. John NT, Blum KA and Hakimi AA: Role of lymph node dissecon in renal cell cancer. Urol Oncol 2019; 37: 187.
279. Gershman B, Thompson RH, Boorjian SA et al: Radical nephrectomy with or without lymph node dissecon for high risk
nonmetastac renal cell carcinoma: A mul-instuonal analysis. J Urol 2018; 199: 1143.
280. Weight CJ, Mulders PF, Pantuck AJ et al: The role of adrenalectomy in renal cancer. Euro Urology Focus 2016; 1: 251.
281. Lane BR, Tiong HY, Campbell SC et al: Management of the adrenal gland during paral nephrectomy. J Urol 2009; 181:
2430.
282. Bratslavsky G, Linehan WM: Surgery: Roune adrenalectomy in renal cancer--an anquated pracce. Nat Rev Urol 2011;
8: 534.
283. Kerbl K, Clayman RV, McDougall EM et al: Laparoscopic nephrectomy: the Washington University experience. Br J Urol
1994; 73: 231.
284. Dunn MD, Pors AJ, Shalhav AL et al: Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol 2000;
164: 1153.
285. Tan H-J, Wolf JS, Ye Z et al: Populaon-level comparave eecveness of laparoscopic versus open radical nephrectomy
for paents with kidney cancer. Cancer 2011; 117: 4184.
286. Mullins JK, Feng T, Pierorazio PM et al: Comparave analysis of minimally invasive paral nephrectomy techniques in the
treatment of localized renal tumors. Urology 2012; 80: 316.
287. Xia L, Wang X, Xu T et al: Systemac review and meta-analysis of comparave studies reporng perioperave outcomes
of robot-assisted paral nephrectomy versus open paral nephrectomy. J Endourol 2016; Epub ahead of print.
288. Wu Z, Li M, Liu B et al: Roboc versus open paral nephrectomy: a systemac review and meta-analysis. PloS one 2014;
9: e94878.

54
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
289. Luciani LG, Porpiglia F, Cai T et al: Operave safety and oncologic outcome of laparoscopic radical nephrectomy for renal
cell carcinoma >7 cm: a mulcenter study of 222 paents. Urology 2013; 81: 1239.
290. Hillyer SP, Bhayani SB, Allaf ME et al: Roboc paral nephrectomy for solitary kidney: a mul-instuonal analysis. Urol-
ogy 2013; 81: 93.
291. Pierorazio PM, Hyams ES, Lin BM et al: Laparoscopic radical nephrectomy for large renal masses: crical assessment of
perioperave and oncologic outcomes of stage T2a and T2b tumors. Urology 2012; 79: 570.
292. Abaza R, Shabsigh A, Castle E et al: Mul-Instuonal experience with roboc nephrectomy with inferior vena cava tu-
mor thrombectomy. J Urol 2016; 195: 865.
293. Kundavaram C, Abreu AL, Chopra S et al. Advances in roboc vena cava tumor thrombectomy: intracaval balloon occlu-
sion, patch graing, and vena cavoscopy. Eur Urol 2016; 70: 884.
294. Auenberg GB, Curry M, Gennarelli R et al: Comparison of cancer specic outcomes following minimally invasive and
open surgical resecon of early stage kidney cancer from a naonal cancer registry. J Urol 2020; 203: 1094.
295. Peyronnet B, Seisen T, Oger E et al: Comparison of 1800 roboc and open paral nephrectomies for renal tumors. Ann
Surg Oncol 2016; 23: 4277.
296. Cacciamani GE, Medina LG, Gill T et al: Impact of surgical factors on roboc paral nephrectomy outcomes: Comprehen-
sive systemac review and meta-analysis. J Urol 2018; 200: 258.
297. Acar C, Bilen C, Bayazit Y et al: Quality of life survey following laparoscopic and open radical nephrectomy. Urol J 2014;
11: 1944.
298. Hyams E, Pierorazio P, Mullins JK et al: A comparave cost analysis of robot-assisted versus tradional laparoscopic par-
al nephrectomy. J Endourol 2012; 26: 843.
299. Laydner H, Isac W, Autorino R et al: Single instuonal cost analysis of 325 roboc, laparoscopic, and open paral ne-
phrectomies. Urology 2013; 81: 533.
300. Hughes D, Camp C, O'Hara J et al: Health resource use aer robot-assisted surgery vs open and convenonal laparoscop-
ic techniques in oncology: analysis of English secondary care data for radical prostatectomy and paral nephrectomy.
BJU Int 2016; 117: 940.
301. Castle SM, Gorbay V, Avallone MA et al: Cost comparison of nephron-sparing treatments for cT1a renal masses. Urol
Oncol 2013; 31: 1327.
302. Mano R, Schulman A, Hakimi AA et al: Cost comparison of open and roboc paral nephrectomy using a short postoper-
ave pathway. Urology 2015; 85: 596.
303. Algaba F, Delahunt B, Berney DM et al: Handling and reporng of nephrectomy specimens for adult renal tumours: a
survey by the European Network of Uropathology. J Clin Pathol 2012; 65: 106.
304. Srigley JR, Amin MB, Campbell SC et al: Protocol for the examinaon of specimens from paents with invasive carcinoma
of renal tubular origin. College of American Pathologists 2017; hp://www.cap.org/web/oracle/webcenter/portalapp/
pagehierarchy/cancer_protocol_templates.jspx?_adf.ctrl-state=15bj4qi2ja_4&_afrLoop=1300546090297309#!.
305. Choueiri TK and Motzer RJ: Systemic therapy for metastac renal-cell carcinoma. N Engl J Med 2017; 376: 354.
306. Monteiro FSM, Soares A, Debiasi M et al: First-line treatment of metastac renal cell carcinoma in the immuno-oncology
era: Systemac review and network meta-analysis. Clin Genitourin Cancer 2020; 18: 244.
307. Chowdhury N and Drake CG: Kidney cancer: An overview of current therapeuc approaches. Urol Clin North Am 2020;
47: 419.
308. Heng DY, Xie W, Regan MM et al: External validaon and comparison with other models of the internaonal metastac
renal-cell carcinoma database consorum prognosc model: A populaon-based study. Lancet Oncol 2013; 14: 141.
309. Wood E, Donin N and Shuch B: Adjuvant therapy for localized high-risk renal cell carcinoma. Urol Clin North Am 2020;
47: 345.
310. Correa AF, Jegede O, Haas NB et al: Predicng renal cancer recurrence: Dening limitaons of exisng prognosc models
with prospecve trial-based validaon. J Clin Oncol 2019; 37: 2062.
311. Agrawal S, Haas NB, Bagheri M et al: Eligibility and radiologic assessment for adjuvant clinical trials in kidney cancer. JA-
MA Oncol 2019;
312. Administraon FaD: Fda approves suninib malate for adjuvant treatment of renal cell carcinoma. 2018. hps://
www.fda.gov/drugs/resources-informaon-approved-drugs/fda-approves-suninib-malate-adjuvant-treatment-renal-
cell-carcinoma. 01/27/2021.
313. Ravaud A, Motzer RJ, Pandha HS et al: Adjuvant suninib in high-risk renal-cell carcinoma aer nephrectomy. N Engl J
Med 2016; 375: 2246.

55
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
314. Olweny EO, Park SK, Tan YK et al: Radiofrequency ablaon versus paral nephrectomy in paents with solitary clinical
t1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 2012; 61:
1156.
315. Thompson RH, Atwell T, Schmit G et al: Comparison of paral nephrectomy and percutaneous ablaon for ct1 renal
masses. Eur Urol 2015; 67: 252.
316. Youn CS, Park JM, Lee JY et al: Comparison of laparoscopic radiofrequency ablaon and open paral nephrectomy in
paents with a small renal mass. Korean J Urol 2013; 54: 603.
317. Tanagho YS, Roytman TM, Bhayani SB et al: Laparoscopic cryoablaon of renal masses: single-center long-term experi-
ence. Urology 2012; 80: 307.
318. Gervais DA, McGovern FJ, Arellano RS et al: Radiofrequency ablaon of renal cell carcinoma: part 1, Indicaons, results,
and role in paent management over a 6-year period and ablaon of 100 tumors. AJR Am J Roentgenol 2005; 185: 64.
319. Best SL, Park SK, Youssef RF et al: Long-term outcomes of renal tumor radio frequency ablaon straed by tumor diam-
eter: size maers. J Urol 2012; 187: 1183.
320. Sidana A, Aggarwal P, Feng Z et al: Complicaons of renal cryoablaon: a single center experience. J Urol 2010; 184: 42.
321. Lehman DS, Hruby GW, Phillips CK et al: First Prize (e): Laparoscopic renal cryoablaon: ecacy and complicaons for
larger renal masses. J Endourol 2008; 22: 1123.
322. Atwell TD, Carter RE, Schmit GD et al: Complicaons following 573 percutaneous renal radiofrequency and cryoablaon
procedures. J Vasc Interv Radiol 2012; 23: 48.
323. Hinshaw JL, Shadid AM, Nakada SY et al: Comparison of percutaneous and laparoscopic cryoablaon for the treatment
of solid renal masses. AJR Am J Roentgenol 2008; 191: 1159.
324. Leveillee RJ, Castle SM, Gorbay V et al: Oncologic outcomes using real-me peripheral thermometry-guided radiofre-
quency ablaon of small renal masses. J Endourol 2013; 27: 480.
325. Ramirez D, Ma YB, Bedir S et al: Laparoscopic radiofrequency ablaon of small renal tumors: long-term oncologic out-
comes. J Endourol 2014; 28: 330.
326. Finley DS, Beck S, Box G et al: Percutaneous and laparoscopic cryoablaon of small renal masses. J Urol 2008; 180: 492.
327. Bandi G, Hedican S, Moon T et al: Comparison of postoperave pain, convalescence, and paent sasfacon aer lapa-
roscopic and percutaneous ablaon of small renal masses. J Endourol 2008; 22: 963.
328. Hegarty NJ, Gill IS, Desai MM et al: Probe-ablave nephron-sparing surgery: cryoablaon versus radiofrequency ablaon.
Urology 2006; 68: 7.
329. Atwell TD, Schmit GD, Boorjian SA et al: Percutaneous ablaon of renal masses measuring 3.0 cm and smaller: compara-
ve local control and complicaons aer radiofrequency ablaon and cryoablaon. AJR 2013; 200: 461.
330. Kunkle DA, Uzzo RG: Cryoablaon or radiofrequency ablaon of the small renal mass. Cancer 2008; 113: 2671.
331. Hong K, Georgiades C: Radiofrequency ablaon: mechanism of acon and devices. J Vasc Interv Radiol 2010; 21:S179.
332. Ahmed M, Brace CL, Lee FT et al: Principles of and advances in percutaneous ablaon. Radiology 2011; 258: 351.
333. Modabber M, Marn J, Athreya S: Thermal versus impedance-based ablaon of renal cell carcinoma: a meta-analysis.
Cardiovasc Intervent Radiol 2014; 37: 176.
334. Lirup PJ, Jallad B, Vorugu V et al: Lethal isotherms of cryoablaon in a phantom study: Eects of heat load, probe size
and number. J Vasc Interv Radiol 2009; 20: 1343.
335. Lirup PJ, Ahmed A, Aoun HD et al: CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 2007; 18:
383.
336. Woolley ML, Schulsinger DA, Durand DB et al: Eect of freezing parameters (freeze cycle and thaw process) on ssue
destrucon following renal cryoablaon. J Endourol 2002; 16: 519.
337. Young JL , McCormick DW, Kolla SB et al: Are mulple cryoprobes addive or synergisc in renal cryotherapy? Urology
2012; 79: 484.
338. Tan YK, Best SL, Olweny EO et al: Radiofrequency ablaon of the incidental benign small renal tumor: outcomes and fol-
low-up protocol. Urology 2012; 79: 827.
339. Lay AH, Faddegon S, Olweny EO et al: Oncologic ecacy of radiofrequency ablaon for small renal masses: histologic
subtype maers. J Urol 2016; 196: 41.
340. Widdershoven CV, Aarts BM, Zondervan PJ et al: Renal biopsies performed before versus during ablaon of t1 renal tu-
mors: Implicaons for prevenon of overtreatment and follow-up. Abdom Radiol (NY) 2020;

56
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
341. Wells SA, Wong VK, Wimann TA et al: Renal mass biopsy and thermal ablaon: Should biopsy be performed before or
during the ablaon procedure? Abdom Radiol (NY) 2017; 42: 1773.
342. Jimenez J, Zhang Z, Zhao J et al: Surgical salvage of thermal ablaon failure for renal cell carcinoma. J Urol 2016; 195:
594.
343. Karam JA, Wood CG, Compton ZR et al: Salvage surgery aer energy ablaon for renal masses. BJU Int 2015; 115: 74.
344. Nuall M, van der Meulen J, Emberton M: Charlson scores based on ICD-10 administrave data were valid in assessing
comorbidity in paents undergoing urological cancer surgery. J Clin Epidemiol 2006; 59: 265.
345. Lascano D, Pak JS, Kates M et al: Validaon of a frailty index in paents undergoing curave surgery for urologic malig-
nancy and comparison with other risk stracaon tools. Urol Oncol 2015; 33: 426.
346. Klae T, Ficarra V, Gratzke C et al: A literature review of renal surgical anatomy and surgical strategies for paral ne-
phrectomy. Eur Urol 2015; 68: 980.
347. Bilimoria KY, Liu Y, Paruch JL et al: Development and evaluaon of the universal ACS NSQIP surgical risk calculator: a de-
cision aid and informed consent tool for paents and surgeons. J Am Coll Surg 2013; 217: 833.
348. Charles C, Gafni A, Whelan T: Decision-making in the physician-paent encounter: revising the shared treatment deci-
sion-making model. Soc Sci Med 1999; 49: 651.
349. Crispen PL, Viterbo R, Boorjian SA et al: Natural history, growth kinecs, and outcomes of untreated clinically localized
renal tumors under acve surveillance. Cancer 2009; 115: 2844.
350. Kunkle DA, Crispen PL, Chen DY et al: Enhancing renal masses with zero net growth during acve surveillance. J Urol
2007; 177: 849.
351. Crispen PL, Viterbo R, Fox EB et al: Delayed intervenon of sporadic renal masses undergoing acve surveillance. Cancer
2008; 112: 1051.
352. Gupta M, Blute ML, Jr., Su LM et al: Delayed intervenon of small renal masses on acve surveillance. J Kidney Cancer
VHL 2017; 4: 24.
353. Whelan EA, Mason RJ, Himmelman JG et al: Extended duraon of acve surveillance of small renal masses: A prospec-
ve cohort study. J Urol 2019; 202: 57.
354. Campi R, Sessa F, Cor F et al: Triggers for delayed intervenon in paents with small renal masses undergoing acve
surveillance: A systemac review. Minerva Urol Nefrol 2020; 72: 389.
355. Mehrazin R, Smaldone MC, Egleston B et al: Is anatomic complexity associated with renal tumor growth kinecs under
acve surveillance? Urol Oncol 2015; 33: 167.
356. Lane BR, Abouassaly R, Gao T et al: Acve treatment of localized renal tumors may not impact overall survival in paents
aged 75 years or older. Cancer 2010; 116: 3119.
357. Kawaguchi S, Fernandes KA, Finelli A et al: Most renal oncocytomas appear to grow: Observaons of tumor kinecs with
acve surveillance. J Urol 2011; 186: 1218.
358. Uzosike AC, Patel HD, Alam R et al: Growth kinecs of small renal masses on acve surveillance: Variability and results
from the dissrm registry. J Urol 2018; 199: 641.
359. Finelli A, Cheung DC, Al-Matar A et al: Small renal mass surveillance: Histology-specic growth rates in a biopsy-
characterized cohort. Eur Urol 2020; 78: 460.
360. Richard PO, Jewe MA, Bha JR et al: Acve surveillance for renal neoplasms with oncocyc features is safe. J Urol
2016; 195: 581.
361. Crispen PL, Wong YN, Greenberg RE et al: Predicng growth of solid renal masses under acve surveillance. Urol Oncol
2008; 26: 555.
362. Surgeons ACo: Acs risk calculator 2021. hps://riskcalculator.facs.org/RiskCalculator/. 01/27/2021.
363. Motzer RJ, Bacik J, Mariani T et al: Treatment outcome and survival associated with metastac renal cell carcinoma of
non-clear-cell histology. J Clin Oncol 2002; 20: 2376.
364. Heng DY, Xie W, Regan MM et al: Prognosc factors for overall survival in paents with metastac renal cell carcinoma
treated with vascular endothelial growth factor-targeted agents: Results from a large, mulcenter study. J Clin Oncol
2009; 27: 5794.
365. Lee SE, Byun SS, Han JH et al: Prognosc signicance of common preoperave laboratory variables in clear cell renal cell
carcinoma. BJU Int 2006; 98: 1228.
366. Bos SD, Piers DA and Mensink HJ: Roune bone scan and serum alkaline phosphatase for staging in paents with renal
cell carcinoma is not cost-eecve. Eur J Cancer 1995; 31a: 2422.

57
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
367. Kriteman L and Sanders WH: Normal alkaline phosphatase levels in paents with bone metastases due to renal cell carci-
noma. Urology 1998; 51: 397.
368. Grünwald V, Eberhardt B, Bex A et al: An interdisciplinary consensus on the management of bone metastases from renal
cell carcinoma. Nat Rev Urol 2018; 15: 511.
369. Koga S, Tsuda S, Nishikido M et al: The diagnosc value of bone scan in paents with renal cell carcinoma. J Urol 2001;
166: 2126.
370. Young RJ, Sills AK, Brem S et al: Neuroimaging of metastac brain disease. Neurosurgery 2005; 57: S10.
371. Brem S and Panal JG: An era of rapid advancement: Diagnosis and treatment of metastac brain cancer. Neurosurgery
2005; 57: S5.
372. Suarez-Sarmiento A, Jr., Nguyen KA, Syed JS et al: Brain metastasis from renal-cell carcinoma: An instuonal study. Clin
Genitourin Cancer 2019; 17: e1163.
373. Liu Y: The place of fdg pet/ct in renal cell carcinoma: Value and limitaons. Front Oncol 2016; 6: 201.
374. Fuccio C, Ceci F, Castellucci P et al: Restaging clear cell renal carcinoma with 18f-fdg pet/ct. Clin Nucl Med 2014; 39:
e320.
375. Alongi P, Picchio M, Zaoni F et al: Recurrent renal cell carcinoma: Clinical and prognosc value of fdg pet/ct. Eur J Nucl
Med Mol Imaging 2016; 43: 464.
376. Wang HY, Ding HJ, Chen JH et al: Meta-analysis of the diagnosc performance of [18f]fdg-pet and pet/ct in renal cell
carcinoma. Cancer Imaging 2012; 12: 464.
377. University R: Zirconium-89-girentuximab pet/ct imaging in renal cell carcinoma. 2016. hps://www.clinicaltrials.gov/ct2/
show/NCT02883153. 02/04/2021.
378. Psutka SP and Master VA: Role of metastasis-directed treatment in kidney cancer. Cancer 2018; 124: 3641.
379. Dabestani S, Beisland C, Stewart GD et al: Long-term outcomes of follow-up for inially localised clear cell renal cell car-
cinoma: Recur database analysis. Eur Urol Focus 2019; 5: 857.
380. Borregales LD, Kim DY, Staller AL et al: Prognoscators and outcomes of paents with renal cell carcinoma and adjacent
organ invasion treated with radical nephrectomy. Urol Oncol 2016; 34: 237.e19.
381. Yu KJ, Keskin SK, Meissner MA et al: Renal cell carcinoma and pathologic nodal disease: Implicaons for american joint
commiee on cancer staging. Cancer 2018; 124: 4023.
382. Merrill MM, Wood CG, Tannir NM et al: Clinically nonmetastac renal cell carcinoma with sarcomatoid dedierenaon:
Natural history and outcomes aer surgical resecon with curave intent. Urol Oncol 2015; 33: 166.e21.
383. Zhang BY, Thompson RH, Lohse CM et al: A novel prognosc model for paents with sarcomatoid renal cell carcinoma.
BJU Int 2015; 115: 405.
384. Merrill SB, Sohl BS, Hamirani A et al: Capturing renal cell carcinoma recurrences when asymptomac improves paent
survival. Clin Genitourin Cancer 2019; 17: 132.
385. Thomas AZ, Adibi M, Borregales LD et al: Surgical management of local retroperitoneal recurrence of renal cell carcino-
ma aer radical nephrectomy. J Urol 2015; 194: 316.
386. Beisland C, Guðbrandsdor G, Reisæter LA et al: A prospecve risk-straed follow-up programme for radically treated
renal cell carcinoma paents: Evaluaon aer eight years of clinical use. World J Urol 2016; 34: 1087.
387. Donat SM, Diaz M, Bisho JT et al: Follow-up for clinically localized renal neoplasms: Aua guideline. J Urol 2013; 190:
407.
388. Levy DA, Slaton JW, Swanson DA et al: Stage specic guidelines for surveillance aer radical nephrectomy for local renal
cell carcinoma. J Urol 1998; 159: 1163.
389. Karam JA and Wood CG: The role of surgery in advanced renal cell carcinoma: Cytoreducve nephrectomy and metas-
tasectomy. Hematol Oncol Clin North Am 2011; 25: 753.
390. Matsumoto ED, Watumull L, Johnson DB et al: The radiographic evoluon of radio frequency ablated renal tumors. J
Urol 2004; 172: 45.
391. Ganz PA, Hasse MJ, Miller DC: Challenges and opportunies in delivering high-quality cancer care: A 2016 update. Am
Soc Clin Oncol Educ Book 2016; 35: e294.
392. Peabody H, Patel A, Johnson A et al: Development of a novel scoring system quanes opportunies to reduce surgery
for benign renal neoplasms: A retrospecve quality improvement analysis within the music-kidney collaborave. J Urol
2020; 204: 1160.
393. Lubner MG: Radiomics and arcial intelligence for renal mass characterizaon. Radiol Clin North Am 2020; 58: 995.

58
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
394. Farber NJ, Wu Y, Zou L et al: Challenges in RCC imaging: renal insuciency, post-operave surveillance, and the role of
radiomics. Kidney Cancer J 2015; 13: 84.
395. Gorin MA, Rowe SP, Allaf ME: Nuclear imaging of renal tumours: a step towards improved risk stracaon. Nat Rev
Urol 2015; 12: 445.
396. Takahashi M, Rhodes DR, Furge KA et al: Gene expression proling of clear cell renal cell carcinoma: gene idencaon
and prognosc classicaon. Proc Natl Acad Sci USA 2001; 98: 9754.
397. Durinck S, Stawiski EW, Pavia-Jimenez A et al: Spectrum of diverse genomic alteraons dene non-clear cell renal carci-
noma subtypes. Nat Genet 2015; 47: 13.
398. Linehan WM and Rickes CJ: The cancer genome atlas of renal cell carcinoma: Findings and clinical implicaons. Nat Rev
Urol 2019; 16: 539.
399. Linehan WM, Spellman PT, Rickes CJ et al: Comprehensive molecular characterizaon of papillary renal-cell carcinoma.
N Engl J Med 2016; 374: 135.
400. Cancer Genome Atlas Research Network: Comprehensive molecular characterizaon of clear cell renal cell carcinoma.
Nature 2013; 499: 43.
401. Cancer Genome Atlas Research Network, Linehan WM, Spellman PT et al: Comprehensive molecular characterizaon of
papillary renal-cell carcinoma. N Engl J Med 2016; 374: 135.
402. Davis CF, Rickes CJ, Wang M et al: The somac genomic landscape of chromophobe renal cell carcinoma. Cancer Cell
2014; 26: 319.
403. Nel I, Gauler TC, Bublitz K et al: Circulang tumor cell composion in renal cell carcinoma. PLoS One 2016; 11: e0153018.
404. Ellinger J, Gevensleben H, Muller SC et al: The emerging role of non-coding circulang RNA as a biomarker in renal cell
carcinoma. Expert Rev Mol Diagn 2016: 16: 1059.
405. Clark DJ, Dhanasekaran SM, Petralia F et al: Integrated proteogenomic characterizaon of clear cell renal cell carcinoma.
Cell 2019; 179: 964.
406. Gorin MA, Verdone JE, van der Toom E et al: Circulang tumour cells as biomarkers of prostate, bladder, and kidney can-
cer. Nat Rev Urol 2017; 14: 90.
407. Lin GA, Fagerlin A: Shared decision making: state of the science. Circ Cardiovasc Qual Outcomes 2014; 7: 328.
408. Wieman HO, Dansokho SC, Colquhoun H et al: User-centered design and the development of paent decision aids:
protocol for a systemac review. Syst Rev 2015; 4: 11.
409. Suk-Ouichai C, Tanaka H, Wang Y et al: Renal cancer surgery in paents without preexisng chronic kidney disease-is
there a survival benet for paral nephrectomy? J Urol 2019; 201: 1088.
410. Gershman B, Thompson RH, Boorjian SA et al: Radical versus paral nephrectomy for ct1 renal cell carcinoma. Eur Urol
2018; 74: 825.
411. Campbell SP, Song DY, Pierorazio PM et al: Stereotacc ablave radiotherapy for the treatment of clinically localized
renal cell carcinoma. J Oncol 2015; 2015: 547143.
412. Maloney E, Hwang JH: Emerging HIFU applicaons in cancer therapy. Int J Hyperthermia 2015; 31: 302.
413. Lele PP, Pierce AD: The thermal hypothesis of the mechanism of ultrasonic focal destrucon in organized ssues. Inter-
acons of ultrasound and biological ssues - workshop proceedings. U.S.D.H.E.W. Publicaon (FDA); 1972.
414. Vallancien G, Charer-Kastler E, Harouni M et al: Focused extracorporeal pyrotherapy: experimental study and feasibility
in man. Semin Urol 1993; 11: 7.
415. Kohrmann KU, Michel MS, Gaa J et al: High intensity focused ultrasound as noninvasive therapy for mullocal renal cell
carcinoma: case study and review of the literature. J Urol 2002; 167: 2397.
416. Marberger M, Schatzl G, Cranston D et al: Extracorporeal ablaon of renal tumours with high-intensity focused ultra-
sound. BJU Int 2005; 95: Suppl 2:52.
417. Hacker A, Michel MS, Marlinghaus E et al: Extracorporeally induced ablaon of renal ssue by high-intensity focused
ultrasound. BJU Int 2006; 97: 779.
418. Moore C, Salas N, Zaias J et al: Eects of microwave ablaon of the kidney. J Endourol 2010; 24: 439.
419. Staord RJ, Fuentes D, Ellio AA et al: Laser-induced thermal therapy for tumor ablaon. Crit Rev Biomed Eng 2010; 38:
79.
420. Geman MT, Lotan Y, Lindberg G et al: Laparoscopic intersal laser coagulaon of renal ssue with and without hilar
occlusion in the porcine model. J Endourol 2002; 16: 565.
421. Williams JC, Swischuk PN, Morrison PM et al: Laser-induced thermotherapy of renal cell carcinoma in man: dosimetry,
ultrasound, and histopathologic correlaon. Proc. SPIE 2000.

59
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
Copyright © 2021 American Urological Association Education and Research, Inc.®
422. Rusn GJ, Mead GM, Stenning SP et al: Randomized trial of two or ve computed tomography scans in the surveillance
of paents with stage i nonseminomatous germ cell tumors of the tess: Medical research council trial te08, isrct-
n56475197--the naonal cancer research instute tess cancer clinical studies group. J Clin Oncol 2007; 25: 1310.
423. Rosa G, Ambrosini G, Barni S et al: A randomized trial of intensive versus minimal surveillance of paents with resected
dukes b2-c colorectal carcinoma. Ann Oncol 2016; 27: 274.
424. Liu W, Mu S, Yao J et al: Analycal and clinical validaon of a next-generaon sequencing-based circulang tumor DNA
(ctDNA) assay assures its clinical applicaon. Ann Oncol 2017; 28: v449-v452.

60
Renal Mass and Localized Renal Cancer: Evalua-
tion, Management, and Follow-Up Panel, Consult-
ants, and Staff 2021
Steven Campbell, MD (Chair)
Cleveland Clinic Foundation
Cleveland, OH
Robert G. Uzzo, MD
Fox Chase Cancer Center
Philadelphia, PA
Peter Earl Clark, MD
Atrium Health
Charlotte, NC
Sam S. Chang, MD
Vanderbilt University Medical Center
Nashville, TN
Jose A. Karam, MD
MD Anderson Cancer Center
Houston, TX
Consultants
Lesley Souter, PhD
Staff
Marybeth Farquhar, PhD, MSN, RN
Erin Kirkby, MS
Leila Rahimi, MHS
Brooke Bixler, MPH
Emily Calvert, MSN, RN
CONFLICT OF INTEREST DISCLOSURES
All panel members completed COI disclosures. Disclo-
sures listed include both topic– and non-topic-related
relationships.
Consultant/Advisor: Sam S. Chang, MD, MBA:
GLG, Janssen, BMS, Pfizer, Urogen, Virtuoso Surgical,
mIR; Peter E. Clark, MD: Galil Medical, Merck; Jose A.
Karam: Merck, P fizer; Robert G. Uzzo, MD: UroGen
Pharma, Amgen
Scientific Study or Trial: Sam S. Chang, MD, MBA:
NIH; Jose A. Karam, MD: Roche/Genentech, Mirati;
Robert G. Uzzo, MD: Pfizer, Genentech
Investment Interest: Jose A. Karam, MD: MedTek,
Allogene, Romtech
Health Publishing: Sam S. Chang, MD, MBA: Uro
Today; Jose A. Karam, MD: Frontiers in Genitourinary
Oncology, Annals of Surgical Oncology, Cancer, Clinical
Genitourinary Cancer
Meeting Participant or Lecturer: Robert G. Uzzo,
MD: Janssen
Peer Reviewers 2021
We are grateful to the persons listed below who con-
tributed to the Guideline by providing comments during
the peer review process. Their reviews do not neces-
sarily imply endorsement of the Guideline.
AUA Reviewers (Board of Directors, Science and
Quality Council, Practice Guidelines Committee,
Journal of Urology):
Linda Baker, MD
Thomas Chi, MD
John Denstedt, MD
Martin K. Dineen, MD
James A. Eastham, MD
David A. Ginsberg, MD
David F. Green, MD
Melissa R. Kaufman, MD
Louis R. Kavoussi, MD
Barry A. Kogan, MD
Matthew Edward Nielsen, MD
Phillip M. Pierorazio, MD
Anthony Y. Smith, MD
Thomas Stringer, MD
Raju Thomas, MD
External Reviewers (Non-AUA Affiliates):
Jeffrey A. Cadeddu, MD
Anthony Chang, MD
Sherri M. Donat, MD
Brian R. Lane, MD
Kirill Shiranov, MD
Darius Unwala, MD
Renal Mass and Localized Renal Cancer Panel,
Consultants, and Staff 2017
Steven Campbell, MD (Chair)
Cleveland Clinic Foundation
Cleveland, OH
Robert G. Uzzo, MD (Vice Chair)
Fox Chase Cancer Center
Philadelphia, PA
Mohamad E. Allaf, MD
Johns Hopkins University School of Medicine
Baltimore, MD
Jeffrey A. Cadeddu, MD
UT Southwestern
Dallas, TX
Anthony Chang, MD
University of Chicago
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

61
Chicago, IL
Peter Earl Clark, MD (PGC Rep)
Vanderbilt University Medical Center
Nashville, TN
Brian J. Davis, MD, PhD
Mayo Clinic, Department of Radiation Oncology
Rochester, MN
Ithaar H. Derweesh, MD
University of California San Diego
La Jolla, CA
Leo Giambarresi, PhD (Pt. Advocate)
Debra A. Gervais, MD
Massachusetts General Hospital
Boston, MA
Susie L. Hu, MD
University Medicine
Providence, RI
Brian R. Lane, MD, PhD
Spectrum Health Medical Group - Urology
Grand Rapids, MI
Bradley C. Leibovich, MD, FACS
Mayo Clinic, Department of Urology
Rochester, MN
Phillip M. Pierorazio, MD
Johns Hopkins University School of Medicine
Baltimore, MD
Consultants
Eric B. Bass, MD, MPH
Johns Hopkins Medicine
Baltimore, MD
Staff
Heddy Hubbard, PhD, MPH, RN, FAAN
Abid Khan, MHS, MPP
Erin Kirkby, MS
Shalini Selvarajah, MD
Nenellia K. Bronson, MA
Leila Rahimi, MHS
Brooke Bixler, MPH
CONFLICT OF INTEREST DISCLOSURES
All panel members completed COI disclosures. Disclo-
sures listed include both topic– and non-topic-related
relationships.
Consultant/Advisor: Jeffrey A. Cadeddu, Levita
Magnetics; Peter E. Clark, Galil Medical, Genentech;
Phillip. M. Pierorazio, Myriad Genetics
Meeting Participant or Lecturer: Anthony Chang,
Alexion Pharmaceuticals; Robert G. Uzzo, Janssen
Scientific Study or Trial: Jeffrey A. Cadeddu, Levi-
ta Magnetics; Ithaar H. Derweesh, GalxoSmithKline,
Inc., Pfizer, Inc.
Leadership Position: Brian J. Davis, American Col-
lege of Radiology, American Board of Radiology; Leo I.
Giambarresi, ZERO-The End of Prostate Cancer
Investment Interest: Jeffrey A. Cadeddu, Titan
Medical Inc., Transenterix; Brian J. Davis, Pfizer Inc.
Health Publishing: Anthony Chang, Elsevier
Other: Leo I. Giambarresi, SAR International Inc.
Follow-up for Clinically Localized Renal Neo-
plasms Panel, Consultants and Staff 2013
Sherri Machele Donat, MD (Chair)
Memorial Sloan-Kettering Cancer Center
New York, NY
Sam S. Chang, MD (Vice-Chair)
Vanderbilt University Medical Center
Nashville, TN
Jay Todd Bishoff, MD
Intermountain Medical Group
Salt Lake City, UT
Jonathan A. Coleman, MD
Memorial Sloan-Kettering Cancer Center
New York, NY
Philipp Dahm MD, MHSc
University of Florida
Gainesville, FL
Ithaar H. Derweesh, MD
UCSD Moores Cancer
La Jolla, CA
S. Duke Herrell III, MD
Vanderbilt University Medical Center
Nashville, TN
Susan Hilton, MD
Hospital of the University of Pennsylvania
Philadelphia, PA
Eric Jonasch, MD
University of Texas MD Anderson Cancer Center
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

62
Houston, TX
Daniel Wei Lin, MD
University of Washington
Seattle, WA
Victor Edward Reuter, MD
Memorial Sloan-Kettering– Pathology
New York, NY
Consultants
Mieya Diaz, PhD
CONFLICT OF INTEREST DISCLOSURES
All panel members completed COI disclosures. Relation-
ships that have expired (more than one year old) since
the panel’s initial meeting, are listed. Those marked
with (C) indicate that compensation was received; rela-
tionships designated by (U) indicate no compensation
was received.
Board Member, Officer or Trustee: Victor E. Reu-
ter, United States and Canadian Academy of P a-
thology (U) (Expired).
Consultant/Advisor: Sam S. Chang, Allergan (C)
(Expired), Amgen (C) (Expired), Astellas (C), Centocor
Ortho Biotech (C) (Expired), Dendreon (C), ENDO (C)
(Expired), GE Health Services (C) (Expired), Predictive
Biosciences (C), Sanofi-Aventis (C) (Expired), Janssen
(C) (Expired); Jay Todd Bishoff, Perc Systems (C)
(Expired); Jonathan Coleman, Endocare (C) (Expired);
Ithaar H. Derweesh, Angiodynamics, I nc. (C)
(Expired), Covidien, Inc. (C) (Expired), Cryolife, Inc.
(C) (Expired), Ethicon Endo-Surgery, Inc. (C) (Expired),
GlaxoSmithKline, Inc. (U); S. Duke Herrell, Aesculap
Inc. (C), Covidien Surgical Devices (C) (Expired); Eric
Jonasch, Aveo (C), Bayer P harmaceuticals (C),
Bristol Myers Squibb (C), Genentech (C), Glaxo Smith
Kline (C), Novartis (C), Pfizer (C), Wyeth (C) (Expired);
Daniel Lin, Caris Life Sciences (U) (Expired), Den-
dreon Corporation (C), GenProbe (U), Myriad (C), Pfizer
(C);
Meeting Participant or Lecturer: Sam S. Chang,
Janssen (C); Jay Todd Bishoff, Pfizer (C) (Expired);
Daniel Lin, Dendreon Corporation (C), Myriad (C). In-
vestment Interest: S. Duke Herrell, Veran Medical
Technologies (U).
Scientific Study or Trial: Jay Todd Bishoff, P fizer
(C); Jonathan Coleman, Steba (U); Philipp Dahm,
CureVac (C) (Expired); S. Duke Herrell, Galil Medical
(C), Wilex (C) (Expired); Eric Jonasch, Aveo (C), Bristol
Myers Squibb (C), Glaxo Smith Kline (C), Novartis (C),
Pfizer (C) (Expired); Daniel Lin, Department of Defense
(C), GenProbe (U), NIH/NCI (C), Veteran's Affairs (U).
Other, Employee: Mireya Diaz, Henry Ford Hospi-
tal - Vattikuti Urology Institute (C).
Other: Speaker's Bureau: Eric Jonasch, LW yeth
(C) (Expired); Pfizer (C) (Expired)
Peer Reviewers
We are grateful to the persons listed below who con-
tributed to the Guideline by providing comments during
the peer review process. Their reviews do not neces-
sarily imply endorsement of the Guideline.
Peter C. Albertsen, MD
Mark Ball, MD
Michael Blute, MD
Stephen Boorjian, MD
Rodney H. Breau, MD
Anthony Corcoran, MD
Paul Crispen, MD
John D. Denstedt, MD
James A. Eastham, MD
Pat Fox Fulgham, MD
William F. Gee, MD
David Ginsberg, MD
David F. Green, MD
Frederick A. Gulmi, MD
Kammi Henriksen, MD
Stephen Jackman, MD
Michael Jewett, MD
Kenar Jhaveri, MD
Melissa R. Kaufman,MD
Louis R. Kavoussi, MD
Raymond Leveillee, MD
Deborah J. Lightner, MD
Kevin R. Loughlin, MD
Viraj Master, MD
Surena Matin, MD
Patrick Hayes McKenna, MD
Randall B. Meacham, MD
Joshua J. Meeks, MD
Adam Metwalli, MD
Ravi Munver, MD
Gladell Paner, MD
Allan Pantuck, MD
Maria Picken, MD
Glenn M. Preminger, MD
Marcus Quek, MD
Jay Raman, MD
Stephen Riggs, MD
Paul Russo, MD
Arthur Sagalowsky, MD
Steven Salvatore, MD
Stephen J. Savage, MD
Roger E. Schultz, MD
Kirill Shiranov, MD
Marc Smaldone, MD
American Urological Association (AUA)
Copyright © 2021 American Urological Association Education and Research, Inc.®
Renal Mass and
Localized Renal Cancer

63
Angela Smith, MD
Thomas F. Stringer, MD
Stephen Strup, MD
Li-Ming Su, MD
Chandru P. Sundaram, MD
Scott K. Swanson, MD
R. Houston Thompson, MD
Luan Truong, MD
Christopher Weight, MD
J. Stuart Wolf, Jr., MD
DISCLAIMER
This document was written by the Renal Mass Guideline
Amendment Panel of the American Urological Associa-
tion Education and Research, Inc., which was created in
2020. The Practice Guidelines Committee (PGC) of the
AUA selected the committee chair. Panel members were
selected by the chair. Membership of the Panel included
specialists in urology and primary care with specific ex-
pertise on this disorder. The mission of the panel was to
develop recommendations that are analysis based or
consensus-based, depending on panel processes and
available data, for optimal clinical practices in the treat-
ment of early stage testicular cancer. Funding of the
panel was provided by the AUA. Panel members re-
ceived no remuneration for their work. Each member of
the panel provides an ongoing conflict of interest disclo-
sure to the AUA, and the Panel Chair, with the support
of AUA Guidelines staff and the PGC, reviews all disclo-
sures and addresses any potential conflicts per AUA’s
Principles, Policies and Procedures for Managing Con-
flicts of Interest. While these guidelines do not neces-
sarily establish the standard of care, AUA seeks to rec-
ommend and to encourage compliance by practitioners
with current best practices related to the condition be-
ing treated. As medical knowledge expands and tech-
nology advances, the guidelines will change. Today
these evidence-based guidelines statements represent
not absolute mandates but provisional proposals for
treatment under the specific conditions described in
each document. For all these reasons, the guidelines do
not pre-empt physician judgment in individual cases.
Treating physicians must take into account variations in
resources, and patient tolerances, needs, and prefer-
ences. Conformance with any clinical guideline does not
guarantee a successful outcome. The guideline text
may include information or recommendations about
certain drug uses (‘off label‘) that are not approved by
the Food and Drug Administration (FDA), or about med-
ications or substances not subject to the FDA approval
process. AUA urges strict compliance with all govern-
ment regulations and protocols for prescription and use
of these substances. The physician is encouraged to
carefully follow all available prescribing information
about indications, contraindications, precautions and
warnings. These guidelines and best practice state-
ments are not intended to provide legal advice about
use and misuse of these substances. Although guide-
lines are intended to encourage best practices and po-
tentially encompass available technologies with suffi-
cient data as of close of the literature review, they are
necessarily time-limited. Guidelines cannot include
evaluation of all data on emerging technologies or man-
agement, including those that are FDA-approved, which
may immediately come to represent accepted clinical
practices. For this reason, the AUA does not regard
technologies or management which are too new to be
addressed by this guideline as necessarily experimental
or investigational.
Copyright © 2021 American Urological Association Education and Research, Inc.®
American Urological Association (AUA)
Renal Mass and
Localized Renal Cancer
