
Specific Drugs
Practical Guidance for the Evaluation and
Management of Drug Hypersensitivity: Specific
Drugs
Ana Dioun Broyles, MD
a
, Aleena Banerji, MD
b
, Sara Barmettler, MD
c
, Catherine M. Biggs, MD
d
,
Kimberly Blumenthal, MD
e
, Patrick J. Brennan, MD, PhD
f
, Rebecca G. Breslow, MD
g
, Knut Brockow, MD
h
,
Kathleen M. Buchheit, MD
i
, Katherine N. Cahill, MD
j
, Josefina Cernadas, MD, iPhD
k
, Anca Mirela Chiriac, MD
l
,
Elena Crestani, MD, MS
m
, Pascal Demoly, MD, PhD
n
, Pascale Dewachter, MD, PhD
o
, Meredith Dilley, MD
p
,
Jocelyn R. Farmer, MD, PhD
q
, Dinah Foer, MD
r
, Ari J. Fried, MD
s
, Sarah L. Garon, MD
t
, Matthew P. Giannetti, MD
u
,
David L. Hepner, MD, MPH
v
, David I. Hong, MD
w
, Joyce T. Hsu, MD
x
, Parul H. Kothari, MD
y
, Timothy Kyin, MD
z
,
Timothy Lax, MD
aa
, Min Jung Lee, MD
bb
, Kathleen Lee-Sarwar, MD, MS
cc
, Anne Liu, MD
dd
, Stephanie Logsdon, MD
ee
,
Margee Louisias, MD, MPH
ff
, Andrew MacGinnitie, MD, PhD
gg
, Michelle Maciag, MD
hh
, Samantha Minnicozzi, MD
ii
,
Allison E. Norton, MD
jj
, Iris M. Otani, MD
kk
, Miguel Park, MD
ll
, Sarita Patil, MD
mm
, Elizabeth J. Phillips, MD
nn
,
Matthieu Picard, MD
oo
, Craig D. Platt, MD, PhD
pp
, Rima Rachid, MD
qq
, Tito Rodriguez, MD
rr
, Antonino Romano, MD
ss
,
Cosby A. Stone, Jr., MD, MPH
tt
, Maria Jose Torres, MD, PhD
uu
, Miriam Verdú,MD
vv
, Alberta L. Wang, MD
ww
,
Paige Wickner, MD
xx
, Anna R. Wolfson, MD
yy
, Johnson T. Wong, MD
zz
, Christina Yee, MD, PhD
aaa
,
Joseph Zhou, MD, PhD
bbb
, and Mariana Castells, MD, PhD
ccc
Boston, Mass; Vancouver and Montreal, Canada; Munich,
Germany; Nashville, Tenn; Porto, Portugal; Montpellier and Paris, France; Chicago, Ill; Charlottesville, Va; Newport Beach, Palo Alto,
and San Francisco, Calif; Cincinnati, Ohio; Al-Kuwait, Kuwait; Catania, Italy; Málaga and Ceuta, Spain
INTRODUCTION
Allergists and clinical immunologists around the world are
increasingly faced with the task of addressing drug allergy and
hypersensitivity due to the increase in drug reactions. Furthermore,
this is often required to maintain patients on first-line therapies,
including antibiotics for those with cystic fibrosis, chemothera-
peutic agents for those with cancer, and mAbs for patients with
chronic inflammatory diseases. The endeavor assumes minor risks
a
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
b
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
c
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
d
Department of Pediatrics, British Columbia Children’s Hospital, University of
British Columbia, Vancouver, Canada
e
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
f
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
g
Division of Sports Medicine, Brigham and Women’s Hospital, Boston, Mass
h
Department of Dermatology and Allergy Biederstein, School of Medicine, Tech-
nical University of Munich, Munich, Germany
i
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
j
Division of Allergy, Pulmonary and Critical Care Medicine, Department of Medi-
cine, Vanderbilt University Medical Center, Nashville, Tenn
k
Allergology and Immunology Service, Centro Hospitalar Universitário de S.João
Hospital, Porto, Portugal
l
Division of Allergy, Department of Pulmonology, Hôpital Arnau d de Villeneuve,
University Hospital of Montpellier, Montpellier, France
m
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
n
Division of Allergy, Department of Pulmonology, Hôpital Arnaud de Villeneuve,
University Hospital of Montpellier, Montpellier, France
o
Department of Anesthesiology and Intensive Care Medici ne, Groupe Hospitalier
Paris-Seine-Saint-Denis, Assistance Publique-Hôpitaux de Paris, Paris, France
p
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
q
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
r
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
s
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
t
Associated Allergists and Asthma Specialists, Chicago, Ill
u
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
v
Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and
Women’s Hospital, Boston, Mass
w
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
x
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
y
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
z
Division of Asthma, Allergy & Immunology, University of Virginia, Charlottes-
ville, Va
aa
Division of Allergy and Inflammation, Beth Israel Deaconess Medical Center,
Boston, Mass
bb
Allergy and Immunology at Hoag Medical Group, Newport Beach, Calif
cc
Channing Division of Network Medicine, Brigham and Women’s Hospital, Bos-
ton, Mass
dd
Division of Allergy / Immunology, Stanford University School of Medicine, Palo
Alto, Calif
ee
Division of Allergy and Immunology, Department of Pediatrics, Cincinnati Chil-
dren’s Hospital Medical Center, Cincinnati, Ohio
ff
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
gg
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
hh
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
Chief Editors: Ana Dioun Broyles, MD, Aleena Banerji, MD, and Mariana Castells, MD, PhD
S16

such as urticaria and major risks that include anaphylaxis and
Stevens-Johnson syndrome. Most of the time these must be
addressed without navigation tools or clear algorithms for the
mechanisms of reactions. There are few standardized skin tests/in
vitro tests for diagnosis and rare validated desensitization protocols.
Drug testing and desensitization should be considered on the basis
of consensus reports (International Consensus [ICON], Interna-
tional Collaboration in Asthma, Allergy and Immunology
[ICALL], Practical Allergy [PRACTALL]) as well as practice pa-
rameters. However, new and targeted drugs that better address
diseases in a precise and personalized fashion are emerging rapidly,
and they induce new, unpredictable, and poorly understood re-
actions. This publication was born out of a grassroots need to
provide the seeds of a new discipline: the understanding, diagnosis,
management, and treatment of drug allergy and hypersensitivity as
a practical clinical endeavor.
Dr Thomas Fleisher embraced this as his presidential theme, as
mentioned in the Introduction section of this supplement. In the
General Concepts article in this supplement, Drs Ana Dioun
Broyles, Aleena Banerji, and Mariana Castells provided the foun-
dational steps in describing the phenotypes, endotypes, and bio-
markers of drug reactions, amplifying the Gell and Coombs
classification and providing practical algorithms for the diagnosis
and management.
1
The lead authors next consulted drug hyper-
sensitivity experts from around the world to precisely define specific
drugs and/or drug classes regarding the phenotypic presentations of
reactions, diagnostic tools such as skin testing, in vitro testing, and
challenges, and the best management and treatment approaches
including desensitization. The numerous authors who contributed
to this section of the supplement have provided the most current
information on and the standards for in vitro and in vivo testing and
desensitization procedures when these exist. Their efforts have
resulted in an accurate compilation of drug hypersensitivity pro-
cedures data that can be applied in a personalized fashion to each
drug-allergic patient.
The hope is that this supplement will provide a user-friendly in-
strument that will be used in clinics, hospitals, wards, and the
emergency department on a daily basis, and that its principles will
guide and increase allergist immunologists’ skills and level of comfort
with a practical approach to drug hypersensitivity. The application of
thestandardsdescribedhereshouldhelpstreamlinetheclinical
practice of drug hypersensitivity and provide the most updated and
safe care to all patients with drug hypersensitivity. It is also hoped that
his resource will be updated every 2 to 3 years with new developments
that arise in the diagnosis and management of drug hypersensitivity
so as to continue to improve the quality and safety of care.
ANTIMICROBIAL AGENTS
Penicillins (by Timothy Lax, MD, and Antonino
Romano, MD)
General.
Penicillins are commonly used to treat infections caused
by both gram-negative and gram-positive organisms. Penicillin al-
lergy is one of the most commonly reported drug allergies, with a
prevalence of 5% to 10%.
2,3
The reported prevalence is higher
among hospitalized patients, at 11% to 15%.
4,5
Individuals with a
history of penicillin hypersensitivity are more likely to receive
alternative antibiotic therapy, which can lead to added expense,
ii
Division of Allergy and Clinical Immunology, Respiratory Medicine, Department
of Pediatrics, University of Virginia, Charlottesville, Va
jj
Division of Allergy, Immunology and Pulmonology, Monroe Carell Jr. Children’s
Hospital at Vanderbilt, Nashville, Tenn
kk
Division of Pulmonary, Critical Care, Allergy, and Sleep, Department of Medicine,
University of California, San Francisco Medical Center, San Francisco, Calif
ll
Division of Allergic Diseases, Mayo Clinic, Rochester, Minn
mm
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
nn
Department of Medicine & Pathology, Microbiology and Immunology, Vanderbilt
University Medical Center, Nashville, Tenn
oo
Division of Allergy and Clinical Immunology, Department of Medicine, Hôpital
Maisonneuve-Rosemont, Université de Montréal , Montréal, Québec, Canada
pp
Division of Immunology, Boston Children’s Hospital, Boston, Mass
qq
Division of Immunology, Boston Children’s Hospital, Boston, Mass
rr
Drug Allergy Department, Al-Rashed Allergy Center, Sulaibikhat, Al-Kuwait,
Kuwait
ss
IRCCS Oasi Maria S.S., Troina, Italy & Fondazione Mediterranea G.B. Morgagni,
Catania, Italy
tt
Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University
Medical Center, Nashville, Tenn
uu
Allergy Unit and Research Group, Hospital Regional Universitario de Málaga,
UMA-IBIMA-BIONAND, ARADyAL, Málaga, Spain
vv
Allergy Unit, Hospital Universitario de Ceuta, Ceuta, Spain
ww
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
xx
Division of Allergy and Clinical Immunology, Brigham and Women’s Hospital,
Boston, Mass
yy
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
zz
Division of Rheumatology, Allergy and Immunology, Massachusetts General
Hospital, Boston, Mass
aaa
Division of Immunology, Boston Children’s Hospital, Boston, Mass
bbb
Division of Allergy/Immunology, Boston Children’s Hospital, Boston, Mass
ccc
Drug hypersensitivity and Desensitization Center, Brigham and Women’s Hos-
pital, Boston, Mass
Conflicts of interest: K. Blumenthal reports grants from the National Institutes of
Health (NIH), CRICO, American Academy of Allergy, Asthma, and Immunology
(AAAAI) Foundation, and Massachusetts General Hospital; and a beta-lactam
allergy clinical decision support tool licensed to Persistent Systems. K. Buchheit
receives royalties from UpToDate and grant support from NIAID. K. N. Cahill
receives grant support from NIAID. A. M. Chi riac was part of the HYCOR
Scientific Advisory Board for biology of drug hypersensitivity. P. Dewachter is on
the advisory board dedicated to “Neuromuscular blocking agents and fast-tracking
anesthesia” for MSD France and receives lecture and travel fees from MSD
France, outside of the submitted work. M. P. Giannetti receives research funding
and salary support from Blueprint Pharmaceuticals, not relevant to this article. J.
T. Hsu reports consulting fees from EBSCO, grants from Vedanta Biosciences,
outside the submitted work. T. Kyin is on an advisory board for Sanofi Regeneron.
M. Louisias reports grants fro m Brigham and Women’s Hospital, Agency for
Healthcare Research and Quality (AHRQ ), and NIH. S. Patil receives royalties
from UpToDate for writing on a different topic and research funding from the NIH
National Institute of Allergy and Infectious Diseases (NIAID) and the Charles H.
Hood Foundation Child Health Grant on a different topic. E. J. Philips reports
grants from NIH (P50GM115305, R01HG010863, R01AI152183, R21AI139021,
U01AI154659) and from the National Health and Medical Research Council of
Australia; receives royalties from UpToDate and consulting fees from Biocryst; is
co-director of IIID Pty Ltd that holds a patent for HLA-B*57:01 testing for
abacavir hypersensitivity; and holds a patent for Detection of Human Leukocyte
Antigen-A*32:01 in connection with Diagnosing Drug Reaction with Eosinophilia
and Systemic Symptoms without any financial remuneration and not directly
related to the submitted work. A. Romano is a consultan t for Diater SA (Leganés,
Spain). C. A. Stone Jr receives funding support from AHRQ (K12HS026395) for
research in risk-stratified management of penicillin allergy. P. Wickner receives
support from a CRICO grant that is not relevant to the content of this article. The
rest of the authors declare that they have no relevant conflicts of interest.
Received for publication August 10, 2020; accepted for publication August 10, 2020.
This article is published as part of a supplement supported by the American Acad-
emy of Allergy, Asthma & Immunology.
2213-2198
Ó 2020 American Academy of Allergy, Asthma & Immunology
https://doi.org/10.1016/j.jaip.2020.08.006
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S17

Abbreviations used
ACD- Allergic contact dermatitis
ADR- Adverse drug reaction
AERD- Aspirin-exacerbated respiratory disease
AGEP- Acute generalized exanthematous pustulosis
ART- Antiretroviral
BAT- Basophil activation test
COPD- Chronic obstructive pulmonary disease
COX- Cyclooxygenase
CYC- Cyclophosphamide
CYP450- Cytochrome p450
DHR- Delayed hypersensitivity reaction
DMCD- Direct mast cell degranulation
DRESS- Drug rash (or reaction) with eosinophilia and systemic
symptoms
EAACI- European Academy of Allergy and Clinical
Immunology
EGFR- Epidermal growth factor receptor
EMB- Ethambutol
ENDA- European Network of Drug Allergy
FDA- Food and Drug Administration
HCV- Hepatitis C virus
HIT- Heparin-induced thrombocytopenia
HMW- High molecular weight
IDT- Intradermal test
IM-ADR- Immunologically mediated adverse drug reaction
INH- Isoniazid
LA- Local anesthetic
LMWH- Low-molecular-weight heparin
MM- Multiple myeloma
MTX- Methotrexate
NMBA- Neuromuscular-blocking agent
NSAID- Nonsteroidal anti-inflammatory drug
PH- Progestogen hypersensitivity
PI- Protease inhibitor
PPI- Proton pump inhibitor
PPL- Penicilloyl-polylysine
PT- Prick test
PZA- Pyrazinamide
RCM- Radiocontrast media
RMS- Red men syndrome
SCAR- Severe cutaneous adverse reaction
SJS- Stevens-Johnson syndrome
SMZ-TMP- Sulfamethoxazole-trimethoprim
SPT- Skin prick test
TB- Tuberculosis
TEN- Toxic epidermal necrolysis
TKI- Tyrosine kinase inhibitor
UFH- Unfractionated heparin
vWD- von Willebrand disease
lengthened hospital stays, and increased risk for resistant or-
ganisms such as vancomycin-resistant Enterococcus, Clostridium
difficile, and methicillin-resistant Staphylococcus aureus.
6,7
Despite
the frequency of reported allergy, avoidance of penicillin is not
necessary in the vast majority of individuals. Approximately 90%
to 95% of patients with a reported penicillin allergy can tolerate a
rechallenge after an appropriate allergy evaluation has been per-
formed.
8,9
The discrepancy between reported and actual peni-
cillin allergy may be explained by the waning of penicillin IgE
antibodies over time or by the misclassification of an adverse
reaction or infectious manifestation as a drug reaction.
10-12
Sensitization to penicillin has been reported to decrease every 10
years, and after 20 years fewer than 1% of patients with initial
clinical symptoms compatible with an allergic reaction continue to
maintain their sensitivity. Therefore, a formal allergy evaluation is
recommended by both North American and European guidelines
to optimize patient management.
13-15
Major symptoms of hypersensitivity. Hypersensitivity
reactions to penicillin are classifiable as immediate or nonimmediate
according to their clinical manifestation, time since the last drug
administration, and the onset of symptoms.
16,17
Immediate re-
actions are predominantly IgE-mediated. They can occur within 6
hours after the last drug administration but typically occur within 1
hourof the firstdose of a new treatmentcourse.
17,18
Symptomsofan
acute hypersensitivity reaction include urticaria, angioedema,
conjunctivitis, respiratory symptoms (rhinitis, bronchospasm,
cough, dyspnea), gastrointestinal symptoms (nausea, vomiting,
diarrhea, abdominal pain), and/or anaphylaxis.
16
Nonimmediate reactions occur more than 1 hour after the initial
drug exposure, and they often develop days to weeks after medication
initiation. Manifestations of nonimmediate reactions include mac-
ulopapular or morbilliform exanthems, particularly during treatment
with amoxicillin or ampicillin. In addition, penicillins can elicit
delayed urticaria/angioedema, exfoliative dermatitis, acute general-
ized exanthematous pustulosis (AGEP), and more severe bullous
exanthems such as Stevens-Johnson syndrome (SJS) and toxic
epidermal necrolysis (TEN). Furthermore, hematologic alterations
may occur with certain penicillins, such as methicillin and ampicillin,
and can cause interstitial nephritis, pneumonitis, hepatitis, and/or
vasculitis with or without signs of serum sickness including joint
involvement. The combination of skin eruptions, visceral involve-
ment, hematologic alteration, fever, and lymphadenopathy is termed
drug-induced hypersensitivity syndrome or drug rash (or reaction)
with eosinophilia and systemic symptoms (DRESS). The pathogenic
mechanisms involved in nonimmediate reactions are heterogeneous.
Allergic maculopapular exanthems are T-cellemediated diseases, in
which drug-specific cytotoxic CD4 T cells migrate into the skin.
These T cells then produce IL-5 and kill keratinocytes that present
MHC class II molecules in a perforin-dependent manner.
19,20
Diagnosis. Based on the clinical history and presenting
symptoms, there are distinct diagnostic approaches for an im-
mediate reaction and for a nonimmediate reaction to penicillin.
For patients with a history of TEN, SJS, DRESS, interstitial
nephritis, or hemolytic anemia, reexposure through either drug
challenge or desensitization is contraindicated, unless there are
special circumstances.
Immediate reactions. Penicillins contain a core bicyclic
structure that is composed of a 4-member
b
-lactam ring and a 5-
member thiazolidine ring along with 1 side chain (R1) (Figure 1).
Although too small to be antigenic in its native state, penicillin gains
immunogenicity by spontaneously degrading and covalently binding
to tissue or serum proteins to form haptens.
21
Approximately 95% of
penicillin degrades to form a penicilloyl complex, which is called the
major determinant.
21,22
The remaining penicillin remains in its
native form (benzylpenicillin) or degrades further to form minor
determinants, which include benzylpenicilloate and benzylpenilloate.
For an immediate reaction to penicillin or another
b
-lactam
antibiotic, an IgE antibody to the common
b
-lactam ring as well
as to a possible side chain must be assessed. A drug challenge test
is the criterion standard for evaluating an IgE-mediated allergy
both to penicillin and to other
b
-lactams. Skin testing and in
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S18 BROYLES ET AL

vitro testing have been developed to identify a sensitization to a
major/minor determinant and/or side chain and decrease the risk
for a positive drug challenge test result.
Penicillin skin testing. For patients with a history concern-
ing for an IgE-mediated reaction to penicillin, or whose past
history is unclear, American and European guidelines recommend
skin testing with both major penicillin antigenic determinants
(penicilloyl-polylysine [PPL]) and minor antigenic determinants
(benzylpenicillin [penicillin G], benzylpenicilloate, and benzylpe-
nilloate).
13,15,23
In the United States, penicillin G is the only
commercially available minor determinant, and it is used in
combination with PPL (PRE-PEN; AllerQuest LLC, West
Hartford, Conn), whereas in Europe, benzylpenicilloyl-octa-
L-
lysine and sodium benzylpenilloate (DAP; Diater, Madrid, Spain)
are available as major and minor determinant, respectively.
8
It has been estimated that skin testing with PPL and penicillin
G, without the use of penilloate and penicilloate, may miss 10% to
20% of penicillin-sensitized subjects.
8,9,24-30
The clinical utility of
penilloate and penicilloate is controversial because studies from
North America have found a comparable negative predictive value
(>95%) between skin testing to PPL and penicillin G only versus
PPL and minor determinant mixture reagent in patients challenged
to penicillin. Skin testing with penicillin G alone without the use of
PPL is not recommended, because up to 70% of patients who have
a positive skin test result react only to PPL, and these patients can
still have a severe reaction.
24
Furthermore, the patient populations who have undergone
skin testing with PPL and penicillin G alone are not comparable
to the ones who had all the reagents used for testing. Hence, it is
not possible to compare the negative predictive value. In addi-
tion, recent or severe historical reactors are not typically included
when only PPL and penicillin G are used for skin testing.
Epicutaneous and intradermal skin testing to PPL is performed
using the commercially available undiluted concentration of 6
10
5
mol/L. A final epicutaneous and intradermal concentration
used for penicillin G ranges between 5,940 and 10,000 U/mL and
between 0.01 and 0.02 M for the other minor determinants (pen-
icilloate and penilloate).
8,13
It is recommended that dilutions use
saline, rather than sterile wate r, to lessen the possibility of
false-positive reactions. A 1:10 or 1:100 dilution of PPL and minor
determinants may be used selectively in patients who have experi-
enced extremely severe reactions. In most guidelines, a positive result
is a wheal having its greatest diameteratleast3mmlargerthanthat
seen with negative control, though some authors suggest a 5-mm
wheal for increased sensitivity, especially for PPL, for which a 5-mm
wheal for prick puncture testing is recommended in the package
insert.
8,13-15,31
Concentrations for penicillin skin testing are pre-
sented in Table I.
Pediatric patients and pregnant women who have experienced
immediate reactions should be evaluated using the diagnostic pro-
tocol discussed earlier. Several studies have confirmed the safety of
skin tests in children, with a rate of 1% to 3% of patients experiencing
systemic reactions to skin testing.
32-34
The negative predictive value
of an allergy evaluation that includes skin tests and drug challenge
tests has been shown to be higher than 97% in children.
34,35
Skin testing was also found to be safe in 56 pregnant women
with group B Streptococcus colonization, with only 2 (4%) women
experiencing a mild reaction during skin testing.
36
An oral chal-
lenge was not performed at the time of the skin testing, but of the
skin testenegative women, 47 (89%) went on to receive a full
therapeutic dose of a penicillin-class antibiotic during the peri-
partum period with no systemic reactions observed.
In vitro tests. Serum-specific IgE assays offer the most common
in vitro method for evaluating immediate reactions to penicillins in
both Europe and the United States. The most widely used
commercial method is the fluoroimmunoassay (ImmunoCAP;
TABLE I. Concentrations for penicillin skin testing
Reagent Epicutaneous Intradermal
Penicilloyl-polylysine (PRE-PEN) 6 10
5
M6 10
5
M
Penicillin G 10,000 units/mL 100 units/mL*
1,000 units/mL*
10,000 units/mL
Penicilloate 0.01-0.02 M 0.01-0.02 M
Penilloate 0.01-0.02 M 0.01-0.02 M
M, mole.
*Optional, based on physician discretion.
FIGURE 1. Basic chemical structures of
b
-lactams. R represents side chains that distinguish a
b
-lactam from other members of the same
class of antibiotics.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S19
Thermo-Fisher, Uppsala, Sweden), which is available for a limited
number of penicillins: penicillin G, penicillin V, amoxicillin, and
ampicillin. The low sensitivity (0%-50%) has limited its use in the
United States and seems to correlate with the severity of the reac-
tion.
37-40
Basophil activation test (BAT) has also been evaluated as a
diagnostic tool for immediate hypersensitivity reactions to
penicillin and other
b
-lactams. Its sensitivity is approximately
50%, with a specificity of more than 90%.
39,41
This test has not
been validated, is not commercially available, and is not rec-
ommended for clinical use at this time.
Drug challenge tests. The negative predictive value for
penicillin skin testing has been shown to be greater than 95% in
North American studies. A small subpopulation of patients re-
mains at risk for a potentially severe hypersensitivity reaction
when rechallenged to penicillin.
8,28
Drug challenge is the diagnostic criterion standard for the
exclusion of an immediate reaction and is recommended after
allergy evaluation when the patient is deemed unlikely to be
allergic to the given drug.
After negative penicillin skin test result, amoxicillin is typically
administered as a drug challenge often as a single full dose and oc-
casionally as 1/10th of the final dose and then the final dose.
Amoxicillin is the penicillin most commonlyused for drug challenges
because it has both immunologically significant core penicillin
structures and potentially significant R-group side chains.
42
Chal-
lenge with the drug that caused the reaction, such as amoxicillin-
clavulanate, may also be considered.
Retesting. Resensitizationisanuncommonoccurrencethatde-
velops in upto 2% of patients after re-treatment with a penicillin.
43-45
The use of parenteral antibiotics may increase the risk.
46
Both North
American and European guidelines suggest that repeat skin testing
may be considered for patients with severe immediate reactions,
especially after parenteral administration, even when a therapeutic
course ofpenicillin has previously been tolerated.
13-15
However, most
patients with a history of penicillin allergy who have undergone
negative skin testing and challenge may receive future courses of
penicilllins without a signific antly increased risk of reactions,
compared with the general population. A recent retrospective study of
32 patients indicated that in patients who report penicillin allergy and
have negative penicillin skin testing result, repeated administration of
intravenous penicillin antibiotics appears safe.
47
Nonimmediate reactions. Both delayed reading of intra-
dermal tests (IDTs) and patch tests have been described as
diagnostic tools for nonimmediate reactions. These tests have not
yet been standardized or validated. In the United States, patch
testing and delayed reading of IDTs are not yet routinely per-
formed, but they are included in European guidelines.
15
Delayed-reading IDTs. IDT is performed using penicillin
G as well as any other suspect penicillins or
b
-lactams. Delayed
skin testing with PPL and other minor determinants has been
found to have limited diagnostic value.
48
Penicillin G is administered at a concentration of 10,000 U/mL,
whereas a concentration of 1 to 20 mg/mL can be used for other
suspect penicillins (eg, ampicillin and amoxicillin).
48
The clinician
should start with epicutaneous prick testing. If results are negative
after 15 to 20 minutes have elapsed, one should proceed next to
IDT.Again, results are read after 20 minutes to assessany immediate
responses. Additional readings of delayed reaction to skin tests are
performed after 48 and 72 hours. Any infiltrated erythema with a
diameter larger than 5 mm is considered to be a positive reaction.
Patch test. A patch test for penicillin and other suspect
penicillins/
b
-lactams is performed using a concentration of 5%
to 10% penicillin in petrolatum. The patch should be worn for
48 hours, with readings 15 minutes after removal of the strips
and again 24 hours later.
The specificity for both delayed reading of IDTs and patch
testing is high (90%-100%), but the sensitivity is less than 50% to
60%.
20,49
Delayed-reading IDTs appear to be more sensitive than
patch testing but may also be less specific.
50,51
Repeat exposure to
the drug is often avoided, but there is limited data on the utility of
using these 2 procedures to assist in the evaluation of noneIgE-
mediated drug reactions.
49,52,53
A recent consensus document
indicated that patch testing and delayed intradermal readings could
be of use for the diagnosis of maculopapular rashes, AGEP, and
DRESS. Although patch testing may be considered in SJS and
TEN, delayed reading of IDT is contraindicated in these 2 con-
ditions.
54
A multicenter study evaluated 134 patients with severe
cutaneous reactions to drugs: 72 with DRESS, 45 with AGEP, and
17 with SJS⁄TEN.
52
Patch tests were first performed with drugs
diluted to 1%, and if the results were negative, they were repeated
with the drug diluted to 30% in petrolatum. Seventy-six partici-
pants (56.7%) had positive patch-test results: 46 (64%) of the 72
with DRESS (of the antibiotics, 8 were positive to amoxicillin, 1 to
dicloxacillin, 2 to ceftriaxone, and 1 to imipenem), 26 (58%) of the
45 with AGEP (7 were positive to amoxicillin and 1 to ceftriaxone),
and 4 (24%) of the 17 with SJS⁄TEN (1 was sensitive to amoxi-
cillin). In patients with negative patch-test results, 4 of 11 patients
with AGEP and 3 of 4 patients with DRESS associated with
b
-
lactams were positive on delayed-reading IDTs.
Drug challenge test. A drug challenge test may be consid-
ered for some nonimmediate reactions. Multiple protocols have
been published for such drug challenge tests.
14,15
A full thera-
peutic dose can be administered on the first day or can be given
incrementally over days to weeks. Some studies suggest that an
additional treatment course for 7 to 10 days after the full ther-
apeutic dose is needed to sufficiently exclude a nonimmediate
reaction.
55,56
A potential caveat of this approach lies in exposing
patients with a history of benign reactions to an unnecessary
therapeutic course of penicillin.
Other penicillins
Aminopenicillins.
Amoxicillin and ampicillin are 2 of the most
commonly prescribed aminopenicillins. Immediate reactions are
IgE-mediated to either the common
b
-lactam structure or the R-
group side chain.
57
Patients who are suspected or found to have a
sensitization to amoxicillin or ampicillin should avoid drugs with
similar or identical R-group side chains (until a formal allergy
evaluation can be performed). These drugs include cefadroxil, cef-
prozil, and cefatrizine (identical side chain shared with amoxicillin
and similar for ampicillin) as well as cephalexin, cefaclor, cephradine,
cephaloglycin, and loracarbef (similar for amoxicillin and identical
for ampicillin).
13,14
The avoidance of cephalosporins with identical/
similar side chains does not hold true if the patient has already
tolerated 1 of them, even if he or she is still avoiding penicillins.
Studies have suggested that amoxicillin sensitivity is more
prevalent among European patients, with up to 50% of patients
with a history of penicillin allergy determined to be sensitized to
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S20 BROYLES ET AL

amoxicillin only, compared with 0% to 6% in North Amer-
ica.
8,26,58
As a result, European guidelines recommend that
amoxicillin skin testing be performed concurrently with peni-
cillin for all patients, whereas testing for amoxicillin is not
consistently performed in North America.
14,15
Unlike skin testing for penicillin, skin testing for amoxicillin and
ampicillin is not validated, and the predictive value is unknown. A
drug challenge test is required to assess for an immediate reaction.
Skin testing can be performed using nonirritatingconcentrations that
can potentially help identify sensitized individuals. Nonirritating
concentrations of amoxicillin range from 3 to 25 mg/mL. Similar to
penicillin-specific IgE, amoxicillin-specific IgE diminishes over time.
One study found that all 24 patients who were found to be skin test
positive after exposure to the drug were skin test negative after 5
years.
10
Because the parenteral form of amoxicillin is not available in
the United States, equivalent concentrations for skin testing cannot
be reliably achieved. For ampicillin, skin testing is performed using a
concentration of 2.5 to 25 mg/mL. Recently, the Food and Drug
Administration (FDA) is evaluating a Penicillin Kit that will contain
amoxicillin.
30
If skin testing result is negative, a drug challenge test is rec-
ommended. If either skin testing result or the oral challenge is
positive, then avoidance or desensitization is recommended.
Carbapenems. Carbapenems (imipenem/cilastatin, mer-
openem, ertapenem) share a common
b
-lactam ring, giving rise to
concern for possible cross-reactivity (Figure 1). Studies over the last
decade, performed either on adults or on children, have demon-
strated an absence or very low (1%) rate of cross-reactivity between
penicillins and carbapenems.
59-65
Nonirritating concentrations for
skin testing to common carbapenems are listed in Table II.
For patients with a history of an immediate reaction and negative
skin testing result to penicillin, carbapenem should prove safe. If
penicillin skin testing is not available or cannot be performed, then a
carbapenem drug challenge test is recommended. For a positive
penicillin skin test result, carbapenem can be administered as a 2-
step graded challenge.
61
If skin testing result is negative, it is
reasonable to administer a single full-dose challenge.
61
Monobactams. Monobactams consist of a monocyclic
b
-lactam ring structure with no adjoining rings (Figure 1).
Currently, the only commercially available monobactam is
aztreonam. Studies have demonstrated a lack of cross-reactivity
between penicillin and aztreonam.
61,66,67
A lack of cross-reac-
tivity has also been demonstrated between cephalosporins and
aztreonam, with the exception of ceftazidime, which shares an
identical side chain.
66
Skin testing is performed with a nonirri-
tating concentration of 2 mg/mL. Aztreonam may be given to
patients with a history of penicillin allergy.
Clavulanate. Clavulanate is a
b
-lactam inhibitor and is
frequently combined with a penicillin such as amoxicillin.
Although uncommon, clavulanate has been reported as a caus-
ative agent for immediate hypersensitivity reactions. Skin testing
with an epicutaneous concentration of clavulanate at 10 mg/mL
and intradermal concentrations of 0.1 and 1 mg/mL was found
to be nonirritating in 1 small case series.
68
Skin testing with
intravenous solutions of amoxicillin-clavulate has also been re-
ported.
69
Given the limited availability of isolated clavulanate/
clavulanic acid and intravenous amoxicillin/clavulanic acid in the
United States, a drug challenge to amoxicillin/clavulanic is rec-
ommended if clavulanate is suspected and the initial drug chal-
lenge to amoxicillin is negative.
Management
High-risk patients.
Individuals with a demonstrated sensi-
tivity to penicillin, either on positive skin testing or on failed
drug challenge tests, should avoid the responsible drug as well as
those that are potentially cross-reactive. It is reasonable to repeat
skin testing if many years have passed since previous skin testing.
If there are no reasonable alternatives, both oral and intravenous
desensitization protocols have been well established in American
and European guidelines.
13,15
Examples of these are provided in
Tables III and IV, respectively.
70,71
Low-risk patients. For patients who are at low risk of an
immediate hypersensitivity reaction, including those with a his-
tory of itching without urticaria, mild maculopapular rash (ie,
less than 1-week duration), or other benign rash, a drug challenge
test may be considered without the use of skin testing.
72
Tools,
such as a recently published clinical pathway for patients with
penicillin allergy, can help guide physicians in deciding whether
TABLE II. Nonirritating concentrations for penicillin and
b
-lactams
Penicillin Nonirritating concentration
Penicillin G
14,79
10,000 units/mL
Aminopenicillins
Amoxicillin
14,21,79
3-25 mg/mL
Ampicillin
14,21,79
2.5-25 mg/mL
b
-Lactamaseeresistant
Nafcillin
80
25
m
g/mL
Carboxypenicillins
Ticarcillin
80
20 mg/mL
Ureidopenicillins
Piperacillin
928
20 mg/mL
Carbapenems
Meropenem
64
1 mg/mL
Imipenm
61,63
0.5-1 mg/mL
Ertapenem
61
1 mg/mL
Monobactam
Aztreonam
61
2 mg/mL
TABLE III. Oral penicillin desensitization protocol*
70
Step Penicillin V (units/mL) mL Units Cumulative dose (units)
1 1,000 0.1 100 100
2 1,000 0.2 200 300
3 1,000 0.4 400 700
4 1,000 0.8 800 1,500
5 1,000 1.6 1,600 3,100
6 1,000 3.2 3,200 6,300
7 1,000 6.4 6,400 12,700
8 10,000 1.2 12,000 24,700
9 10,000 2.4 24,000 48,700
10 10,000 4.8 48,000 96,700
11 80,000 1.0 80,000 176,700
12 80,000 2.0 160,000 336,700
13 80,000 4.0 320,000 656,700
14 80,000 8.0 640,000 1,296,700
*Fifteen-minute intervals between each step. Cumulative dose is 1.3 million units
given over a total of 3h 45 min.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S21
drug challenge, drug sensitization, drug avoidance, or further
allergy evaluation presents the best course of action.
73
Cephalosporins (by Kimberly Blumenthal, MD)
General.
Cephalosporin adverse drug reactions (ADRs) affect
about 0.5% to 2.5% of patients. ADRs include nephropathy and
acquisition of C difficile colitis.
74
Although cephalosporin allergy
is approximately 10-fold less common than penicillin allergy, it
remains one of the most commonly reported drug allergies in the
United States.
75
Major symptoms. Cephalosporins can elicit various hyper-
sensitivity reactions, including IgE-mediated reactions charac-
terized by urticaria, angioedema, rhinitis, bronchospasm, and
anaphylaxis. Notably, cephalosporins, especially cefaclor and
cefprozil, may cause serum sicknesselike reactions.
13
They may
also less frequently lead to severe cutaneous adverse reactions
(SCARs) and isolated eosinophilia.
74,76
Diagnosis
Immediate hypersensitivity skin testing.
Most hyper-
sensitivity reactions to cephalosporins are directed at the R-group
side chain (Figure 2) rather than the core
b
-lactam ring mole-
cule.
77,78
However, it may be useful to also perform penicillin
skin testing when patients present with possible sensitivity to
cephalosporins, particularly those in the earlier generations,
which include the aminocephalosporins (cefaclor, cephalexin,
and cefadroxil; Table V). Although skin testing to native ceph-
alosporins is not standardized, a positive skin test result with a
nonirritating concentration suggests the presence of drug-specific
IgE antibodies.
79,80
A negative skin test result does not rule out
an allergy, and must be followed by an observed graded challenge
(Table VI). Similar to data suggestive of loss of IgE-mediated
hypersensitivity to penicillins, patients with IgE-mediated allergy
to cephalosporins may lose sensitivity over time.
81
Delayed hypersensitivity skin testing. For SCARs, us-
ing the RegiSCAR scoring method for diagnosis is recommended,
and skin biopsy is usually indicated.
82
Patch testing may be useful,
especially for DRESS syndrome and AGEP.
77
Patch tests to
cephalosporins may be performed using a 30% dilution of the drug
in petrolatum (not commercially available in the United States),
with readings at 48 and 72 hours.
52
Management
General/clinical pathways.
For patients with true or re-
ported cephalosporin allergy, management can be challenging,
especially for nonallergist providers. A clinical pathway that in-
corporates both cephalosporin generation (because of
b
-lactam
ring relevance by generation) and cephalosporin side chain
(because of cross-reactivity by side chain/R group) can help direct
safe use of
b
-lactam in the setting of previously reported hy-
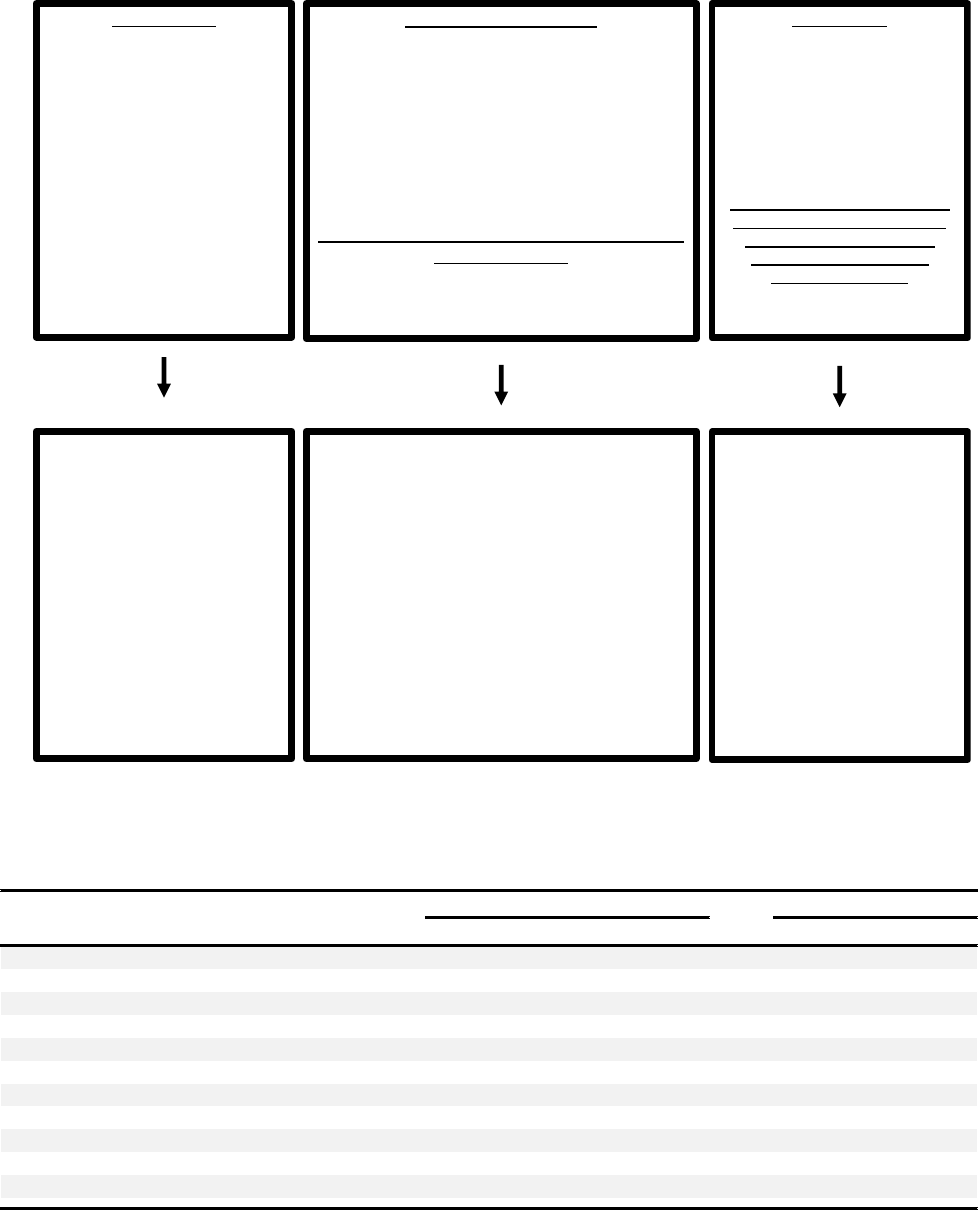
persensitivity, though outcome data are limited (Figure 3, A).
73
Similar pathways have been implemented for demonstration of
cephalosporin tolerance in patients with historical penicillin al-
lergies (Figure 3, B).
73
To date, data on these clinical pathways
confirm that they increase
b
-lactam use in acute therapeutic
situations and increase first-line antibiotic treatment in an
appropriately risk-adverse manner (reactions were observed in
0.5%-4.0% of patients).
73,83
Oral challenge. An oral challenge to cephalosporin would be
appropriate if the reaction appeared to be (1) unlikely to have been
caused by the cephalosporin, (2) not immune-mediated/serious/
life-threatening, or (3) in response to a different cephalosporin with
low to medium risk of cross-reactivity (eg, cephalosporins with
dissimilar side chain, history of IgE-mediated penicillin allergy). As
with any other drug challenge, the potential risks, benefits, and
alternatives should be discussed with the patient and/or the pa-
tient’s parents/guardians and informed consent should be ob-
tained. The procedure should be performed in a monitored clinical
setting where emergency support is readily available. Generally,
drug challenges to dissimilar cephalosporins are safe to perform.
TABLE IV. Example of intravenous desensitization protocol for
b
-lactam dose of 1 g*
13
Solution Volume of diluent (mL) Drug concentration (mg/mL) Total drug to be injected into each bottle (mg)
Solution 1 250 0.04 10
Solution 2 250 0.4 100
Solution 3 250 4 1000
Step Solution Rate (mL/h) Time (min) Administered dose (mg) Cumulative dose (mg)
1 1 2 15 0.02 0.02
2 1 5 15 0.05 0.07
3 1 10 15 0.1 0.17
4 1 20 15 0.2 0.37
5 2 5 15 0.5 0.87
6 2 10 15 1 1.87
7 2 20 15 2 3.87
8 2 40 15 4 7.87
9 3 10 15 10 17.87
10 3 20 15 20 37.87
11 3 40 15 40 77.87
12 3 80 172.9 922.13 1000
Total infusion time 337.9 min
*Modified with permission from Castells.
71
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S22 BROYLES ET AL

Increased caution, warranting skin testingeguided treatment,
choosing alternative agents, or performing a desensitization, may
be indicated for cephalosporin administration with similar side
chains, or for patients with severe reaction histories, or clinically
unstable patients. In cases of serum sicknesselike reaction to
cefaclor or cefprozil, it is appropriate to use another
cephalosporin, preferably one with dissimilar R1 side chains. A
standard 2-step test dose protocol is as safe as longer protocols,
but less likely to induce tolerance (Table IV).
83,84
Desensitization. The most common desensitization protocol
for cephalosporins comprises 12 steps and it is intended for IgE-
mediated reactions (Table IV ).
13
Contraindications to desensi-
tization include severe T-cellemediated reactions such as
DRESS, TEN, or SJS. Although initiating treatment with stan-
dard desensitization protocol is recommended, its duration may
be modified in such a manner that it takes into account a pa-
tient’s relevant history and considers both comorbidities and
acuity of the present illness.
FIGURE 2. Cephalosporin cross-reactivity.
78
b
-Lact am antibiotics can hav e similar or identical R1 or R2 side chains , which may make cross-
reactivity more lik ely. This matrix indicates either a similar (gray) or an identical (red) side chain. Empty box es indicate a lack of side-chain simi larity.
TABLE V. Immediate hypersensitivity cephalosporin skin testing
80,929-931
Test type Cephalexin Cefazolin Cefuroxime Cefotaxime Ceftazidime Ceftriaxone Cefepime* Cefixime
Step 1: Epicutaneous 25 mg/mL 330 mg/mL 100 mg/mL 100 mg/mL 100 mg/mL 100 mg/mL 200 mg/mL 2 mg/mL
Step 2: Intradermal† NAz 3.3 mg/mL 1 mg/mL 1 mg/mL 1 mg/mL 1 mg/mL 2 mg/mL NAz
Step 3: Intradermal NAz 33 mg/mL 10 mg/mL 10 mg/mL 10 mg/mL 10 mg/mL 20 mg/mL NAz
NA, Non-applicable/not available.
Penicillin skin testing may also be appropriate for patients presenting with cephalosporin allergy.
*Nonirritating in clinical practice, MGH Allergy Associates (unpublished data, 2020).
†Optional for patients with history of severe and/or recurrent reactions.
zCephalexin and cefixime are not available as an intravenous preparation.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S23

Sulfamethoxazole-trimethoprim (by Miguel Park, MD)
Epidemiology.
Sulfamethoxazole-trimethoprim (SMZ-TMP)
is a common cause of hypersensitivity and ADRs. Before 1982,
SMZ-TMP was the second most common medication behind
amoxicillin to result in cutaneous ADRs in the hospital.
85
These
reactions are primarily due to hypersensitivity to the sulfonamide
component, and specific hypersensitivity to trimethoprim is rare
and has been reported anecdotally.
86
Patients with HIV have been
reported to have a rate of sensitivity to SMZ-TMP that ranges from
34% to 50%
87,88
; however, the frequency of cutaneous ADRs
exceeds that of those that occur in response to aminopenicillins.
89
Most ADRs to SMZ-TMP are morbilliform, maculopapular
eruptions. IgE-mediated reactions including urticaria/angioe-
dema and anaphylaxis, severe delayed hypersensitivity reactions
(DHRs) such as SJS, TEN, and DRESS, and hepatic, renal, and
hematologic reactions have also been described.
13
Diagnosis. Validated diagnostic testing is not currently avail-
able for SMZ-TMP hypersensitivity.
90
However, if an IgE-medi-
ated ADR to SMZ-TMP is suspected, skin testing using a
nonirritating concentration of 1:100 dilution of 80 mg/mL, or 0.8
mg/mL, may be considered.
80
A positive result could suggest IgE-
mediated sensitization, and a negative result would not rule out an
IgE-mediated reaction to SMZ-TMP. Oral challenge, either as a
single full dose or as 1/10th of the dose followed by the full dose,
may be considered on the basis of clinical history and skin test
results.
91
Dapsone may be tolerated in patients with histories of
sulfonamide reactions; however, there is conflicting information
on cross-reactivity, and avoidance of dapsone is recommended in
patients with histories of severe reactions to sulfonamides.
92
Management. Desensitization is an important component in
the management of SMZ-TMP hypersensitivity. The term
desensitization has traditionally been used for IgE-mediated sensi-
tivity. It has also been applied to treatments for reactions to SMZ-
TMP, even when the mechanisms underlying the reaction are
unclear. The Joint Task Force on Practice Parameters has recom-
mended that the term “temporary induction of tolerance”
13
is
more appropriate than desensitization in these circumstances.
In the event of severe DHRs (SJS, TEN, DRESS, and others)
to SMZ-TMP, the drug should be avoided. If no alternatives
exist and the benefits of treatment with SMZ-TMP outweigh the
risk of death from a severe hypersensitivity reaction,
13
a previ-
ously reported temporary induction of tolerance protocol that
was successfully used for 2 patients may be considered.
93
Most literature on the temporary induction of tolerance to
SMZ-TMP focuses on the HIV patient population. The various
protocols for temporary induction of tolerance to SMZ-TMP
have shown similar success rates (initial success: 80%-90%; long-
term: 60%-80%) (Table VII).
89,94-104
There is no current
consensus on the best protocol for SMZ-TMP temporary in-
duction of tolerance. Interestingly, when patients with a history
of mild to moderate SMZ-TMP hypersensitivity were random-
ized to temporary induction of tolerance or full-dose challenge,
they showed similar success rates in tolerating the drug
(Table VIII). Some studies found full-dose challenge success rates
to range from 58% to 72% and those of temporary induction of
tolerance to range from 60% to 80%.
94,97,102
Leoung et al
98
reported a 75% success rate in the temporary induction of
tolerance group compared with 58% in the full-dose challenge
group (58%) (P ¼ .014). Therefore, a full-dose challenge could
be considered for patients with mild reactions to SMZ-TMP as
an alternative to an induction of tolerance procedure.
Very few studies have examined the temporary induction of
tolerance to SMZ-TMP in non-HIV patient populations.
99,105
Mann et al
99
described 4 patients with a history of SMZ-TMP
hypersensitivity (leukopenia, hives, macular rash, morbilliform
rash) who underwent a successful temporary induction of toler-
ance using either an 8-day protocol or a 22-day protocol.
106,107
Pyle et al
108
reported that 90% of 72 patients with a history of
SMZ-TMP hypersensitivity who required the drug and under-
went temporary induction of tolerance had successful outcomes
with the 6-step, 14-step, or more than 1-day protocols. The data
suggest that SMZ-TMP temporary induction of tolerance may
be considered in non-HIV patients with a history of related
hypersensitivity. The procedure appears to result in success rates
comparable to those seen in patients with HIV.
In summary, temporary induction of tolerance to SMZ-TMP
can be used safely and effectively in patients with and without
HIV who have SMZ-TMP hypersensitivity. For HIV-positive
patients with a history of a mild SMZ-TMP hypersensitivity, a
full-dose challenge can be considered; however, this option may
result in higher rates of ADRs than those associated with the
temporary induction of tolerance. A standardized temporary in-
duction of tolerance protocol for SMZ-TMP is not currently
available, and a range of temporary induction of tolerance pro-
tocols may be appropriate for use with HIV-positive patients
(Table VII). Tables IX, X, and XI present protocols described by
Pyle et al,
108
which offer options for temporary induction of
tolerance to SMZ-TMP for non-HIV patients.
Quinolones (by Maria Jose Torres, MD)
Introduction.
The frequency of hypersensitivity reactions to
quinolones, especially anaphylactic reactions, is increasing, likely
related to the increase in their use. They can induce IgE- and
T–celledependent reactions, with the IgE type being the most
common, and moxifloxacin as an increasing inductor of re-
actions.
109-112
Factors influencing the increase in moxifloxacin IgE
hypersensitivity in countries whereitisprescribed are notknown.
109-
111
A mast cellespecific receptor has been identified (MRGPRX2),
which is a target for direct activation by quinolones and some other
drugs with tetrahydroisoquinoline (THIQ) motifs.
113
A previous
diagnosis of immediate hypersensitivity to
b
-lactams can be a risk
factor for developing IgE reactions to quinolones.
110
Ciprofloxacin is
the main quinolone involved in delayed reactions.
112
Clinical symptoms. The most frequent clinical symptoms
are immediate urticaria and anaphylaxis, with some reports
indicating that they can be severe.
109-112
Delayed reactions
usually reported are maculopapular exanthem, delayed urticaria,
and fixed drug eruptions. Although less frequent, other reactions
such as AGEP, SJS, and TEN have also been described.
Diagnosis. Various factors can complicate the diagnosis of
immediate hypersensitivity reactions to quinolones. First, the
clinical history is often unreliable, because nearly 70% of patients
with a clinical history of quinolone hypersensitivity ultimately
TABLE VI. Cephalosporin drug challenge
73,84
Step 1
1
/
4
of an oral dose/pill/1/10th of parenteral or oral liquid dose,
observe for 30-60 min
Step 2 1 full dose, observe for 60 min
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S24 BROYLES ET AL

can tolerate the drug and are therefore not allergic.
110,112
Second,
skin testing has resulted in a high number of false-positive results,
likely due to the capacity of some quinolones to induce direct
histamine release.
109,110,112,114
Table XII summarizes quinolone
concentrations most frequently recommended in the current
literature.
112,114,115
Therefore, drug challenge is the most useful
diagnostic assay, and procedure-related anaphylaxis is rare in
properly selected patients. Table XIII presents the recommended
procedures for drug challenge tests. Recently, in vitro approaches,
such as radioimmunoassay and BAT, have proved useful tools for
diagnosis, though their sensitivity is not optimal and they are not
commercially available.
109,110,114
The diagnosis of delayed reaction is also difficult, and
particularly relevant is the lack of reliability of the clinical history,
where fewer than 5% of cases evaluated are finally confirmed as
allergic.
110,112
Patch testing has shown high specificity but low
sensitivity. In cases with maculopapular exanthems or delayed
urticaria, the most frequent clinical symptoms, the diagnosis is
usually confirmed with a drug challenge test.
Management. Although there are no general rules for pre-
dicting cross-reactivity, this seems to exist between first- and
second-generation quinolones, with lower levels seen with the
third- and fourth-generation quinolones. Therefore, patients
with immediate hypersensitivity to quinolones are recommended
to avoid the administration of the whole group, and specific
recommendations of tolerance need to be made on a patient-by-
patient basis using a drug challenge test. For higher-risk patients,
desensitization to quinolones may be considered (Table XIV).
However, cross-reactivity in delayed reactions seems to be low.
Macrolides (by Miriam Verdu Benhamu, MD, Anca
Mirela Chiriac, MD, and Pascal Demoly, MD, PhD)
General.
Macrolide antibiotics are considered to be some of
the safest antibiotic treatments available. Their chemical struc-
ture is characterized by a large lactone ring, which can vary from
12 to 16 atoms, with 1 or more sugar chains attached.
116
There
are more than 20 macrolide antibiotics available; erythromycin
Type I (IgE-mediated) HSR
Anaphylaxis
Angioedema
Wheezing or shortness of breath
Laryngeal edema
Hypotension
Hives/urticaria
Unknown reaction with no further details available from
patient/proxy
Mild reaction
Minor rash
(not hives)
Maculopapular rash
(mild Type IV HSR)
Record lists allergy, but patient
denies
Unknown reaction, but patient
denies mucosal involvement,
skin desquamation, organ
involvement, or need for
medical evaluation
OK to:
Use a different generation
cephalosporin and cephalosporin
with dissimilar side chains
†
OR
Use penicillin by
Test Dose Procedure
OR
Use carbapenem
OR
Use alternative agents by microbial
coverage
Type II-IV HSR
Serum sickness
Stevens-Johnson Syndrome
Toxic Epidermal Necrolysis
Acute interstitial nephritis (AIN)
Drug Rash Eosinophilia
Systemic Symptoms (DRESS)
syndrome
Hemolytic anemia
Drug Fever
Avoid using PCNs,
cephalosporins, and
carbapenems
(not amenable to
desensitization)
Use alternative agents by
microbial coverage
If clinical indication for a
beta-lactam, please involve
the Infectious Disease
service and Allergy, if
available
OK to:
Administer 3
rd
/4
th
/5
th
generation cephalosporin if
dissimilar side chains
†
by
Test Dose Procedure
OR
Use carbapenem
OR
Use alternative agents by
microbial coverage
§
OR
If a PCN or a 1
st
/2
nd
generation cephalosporin
is the preferred therapy,
or one of the alternative
agents is substandard,
PCN skin testing is
indicated, call/consult
Allergy, if available
OK to:
Administer PCN or
cephalosporin if dissimilar
side chains
†
by
Test Dose Procedure
OR
Use carbapenem
OR
Use alternative agents by
microbial coverage
§
Reaction to:
1
st
/2
nd
Generation
3
rd
/4
th
Generation
A
FIGURE 3. Cephalosporin hypersensitivity pathway
73
in patients with a history of reported hypersensitivity to (A) cephalosporins and (B)
penicillin. PCN, Penicillin class antibiotic.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S25
OK to:
Use 3
rd
/4th/5th generation cephalosporins by Test
Dose Procedure
OR
Use alternative agent by microbial coverage
OR
Aztreonam or carbapenem
OR
If a PCN or a 1st/2nd generation cephalosporin is
preferred PCN skin testing is indicated, call/
consult Allergy, if available.
Type I (IgE-mediated) HSR
Anaphylaxis
Angioedema
Wheezing or shortness of breath
Laryngeal edema
Hypotension
Hives/urticaria
Unknown reaction with no further details available
from patient/proxy
Mild reaction
Minor rash
(not hives)
Maculopapular rash
(mild Type IV HSR)
Record lists allergy, but patient
denies
Unknown reaction, but patient
denies mucosal involvement,
skin desquamation, organ
involvement, or need for
medical evaluation
OK to:
Use full dose
cephalosporin
OR
Use penicillin by
Test Dose Procedure
OR
Use carbapenem
Type II-IV HSR
Serum sickness
Stevens-Johnson Syndrome
Toxic Epidermal Necrolysis
Acute interstitial nephritis (AIN)
Drug Rash Eosinophilia Systemic
Symptoms (DRESS) syndrome
Hemolytic anemia
Drug Fever
Avoid using PCNs,
cephalosporins, and
carbapenems
(not amenable to
desensitization)
Use alternative agents by
microbial coverage
If clinical indication for a beta-
lactam, please involve the
Infectious Disease service and
Allergy, if available
B
FIGURE 3. (CONTINUED).
TABLE VII. SMZ-TMP temporary induction of tolerance in patients with HIV
Study author/year No. of subjects
Protocol Success rate (%)
Starting dose No. of steps/duration Initial Long- term*
Absar et al,
94
1994 28 2 mg 10/10 d 82% 61%
Gluckstein and Ruskin,
96
1995 22 0.02 mg 6/5 h 86% 71%
Nguyen et al,
100
1995 45 10 ng 40/36 h 82% 60%
Kalanadhabhatta et al,
97
1996 13 2 ng 37/27 h 100% 100%
Caumes et al,
95
1997 48 4 mg 8/3 d 83% 77%
Rich et al,
101
1997 22 20 ng 8/8 d 86% 86%
Ryan et al,
102
1998 13 2 mg 33/33 d 69% 62%
Yoshizawa et al,
104
2000 17 2 mg 10/5 d 88%† 88%
Bonfanti et al,
932
2000 34 10 ng 40/36 h 79% 79%
Leoung et al,
98
2001 97 50 mg 12/6 d 93.8% 75%
Straatmann et al,
103
2002 9 75 mg 11/22 d 60% 60%
*Those who died unrelated to SMZ-TMP and lost to follow-up were counted as part of the successful group.
†Some patients required multiple tries before successful completion.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S26 BROYLES ET AL

was the first macrolide used, and this molecule has 14 atoms in
the lactone ring (14-C), such as clarithromycin and roxi-
thromycin; azithromycin is the only macrolide with 15-C, and
josamycin and spiramycin have 16-C. Macrolide antibiotics
usually have a bacteriostatic effect by inhibiting the mRNA-
direct protein synthesis after binding to the 50S ribosomal
subunit; they are broad-spectrum antibiotics effective against
gram-positive, gram-negative, and atypical pathogens. Their
clinical uses include upper and lower respiratory tract infections,
skin infections, sexually transmitted diseases, and the eradication
regimen of Helicobacter pylori. Their pharmacokinetic properties
include low to moderate oral bioavailability and extensive
diffusion into tissues and fluids due to their lipophilic nature.
The 14-membered ring macrolides have an affinity for cyto-
chrome p450 (CYP450); therefore, potential drug interactions
must be taken into account when other drugs metabolized by
CYP450 enzymes (such as phenytoin, cyclosporine, theophyl-
line, and carbamazepine) are coprescribed.
Major symptoms. Published hypersensitivity reactions to
macrolides include both immediate (<1 hour after drug intake)
and nonimmediate (delayed) reactions.
116-120
Urticaria and/or
angioedema are the most commonly described symptoms, but
maculopapular exanthems (some severe, evocative of DRESS),
fixed drug eruptions, and bullous skin reactions are also seen.
Anaphylaxis caused by macrolides has also rarely been described.
In addition to hypersensitivity reactions, macrolides can induce
gastrointestinal side effects, such as nausea, vomiting, diarrhea, and
abdominal cramps, because they stimulate gut contractility.
116
Macrolides can also cause sensorineural ototoxicity, which is
usually transient, as well as prolongation of the QT interval.
116
Diagnosis. When hypersensitivity to macrolides is suspected,
the most common approach adopted by physicians is avoidance
especially because these agents are rarely indicated as first-line
treatment.
116,121
However, clinical history alone is insufficient to
ascertain the diagnosis, and published series reveal that, after
performing a drug challenge test, hypersensitivity to macrolides is
confirmed in only 2.7% to 17% of cases.
118,120
Therefore, in
some situations, including the prevention/treatment of Toxo-
plasma gondii, eradication of H pylori, and treatment of some
atypical Mycobacteria , testing should be performed.
A detailed clinical history including the macrolide involved, the
chronology, and type of reaction, as well as the treatment used, is
always needed.
Skin tests (prick and sequenced IDT for immediate reactions with
the few available injectable forms and delayed-reading IDTs and
patch tests for nonimmediate reactions) can be helpful in the diag-
nostic evaluation. They are not fully validated, because it is necessary
to perform them in a large number of patients with proper controls,
and therefore, real predictive values are unknown. In a study by
Empedrad et al,
80
concentrations for intradermal testing with
erythromycin and azithromycin were tested in 25 healthy subjects
TABLE IX. SMZ-TMP temporary induction of tolerance short
protocol*
Steps Dose of SMZ-TMP
1 0.02 mg/0.004 mg
2 0.2 mg/0.04 mg
3 2 mg/0.4 mg
4 20 mg/4 mg
5 200 mg/40 mg
6 Final dose
Single (SS): 400 mg/80 mg PO or Double (DS):
800 mg/160 mg PO
PO, per os (by mouth).
*Dosing intervals are scheduled 15, 30, or 60 min apart. Depending on the dosing
interval, the test will take 2 h at 15-min intervals, 3
1
/
2
h at 30-min intervals, and 6
1
/
2
h
at 60-min intervals. Modified from the temporary induction of tolerance protocol by
Gluckstein and Ruskin.
96
TABLE X. SMZ-TMP temporary induction of tolerance long
protocol*
Steps Dose of SMZ-TMP
1 0.08 mg/0.016 mg
2 0.16 mg/0.032 mg
3 0.32 mg/0.064 mg
4 0.64 mg/0.128 mg
5 1.28 mg/0.256 mg
6 2.5 mg/0.512 mg
7 5 mg/1 mg
8 10 mg/2 mg
920mg/4mg
10 40 mg/8 mg
11 80 mg/16 mg
12 160 mg/32 mg
13 320 mg/64 mg
14 440 mg/88 mg
*Dosing interval is 15 min apart. The test will take 4 to 5 h. Modified from the
temporary induction of tolerance protocol by Kalanadhabhatta et al.
97
TABLE XI. SMZ-TMP temporary induction of tolerance 10-
d protocol*
Steps Dose of SMZ-TMP
1 2 mg /0.4 mg
2 4 mg/0.8 mg
3 8 mg/1.6 mg
4 16 mg/3.2 mg
5 40 mg/8 mg
6 80 mg/16 mg
7 160 mg/32 mg
8 320 mg/64 mg
9 400 mg/80 mg
10 800 mg/160 mg
*Dosing interval is daily. The test will take 2 h on the first day and 90 min on
subsequent days. Modified from the temporary induction of tolerance protocol by
Absar et al.
94
TABLE VIII. SMZ-TMP temporary induction of tolerance vs full-
dose challenge
Study author/year
Success rate %
(total no. of subjects in the group)
Temporary induction
of tolerance
Full-dose
challenge
Bonfanti et al,
931
2000 79.5% (34) 72% (25)
Leoung et al,
98
2001 75% (97) 58% (94)
Straatmann et al,
103
2002 60% (9) 60% (9)
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S27
and nonirritating dilutions were set at 0.05 mg/mL and 0.01 mg/
mL, respectively. Mori et al
122
performed an allergy workup in
children with histories of suspected clarithromycin and azi-
thromycin hypersensitivity. Skin tests were performedto these drugs
on both subjects and negative controls. The highest nonirritating
concentrations used for the prick and intradermal tests were 50 mg/
mL and 0.5 mg/mL, respectively, for clarithromycin and 100 mg/
mL and 0.01 mg/mL, respectively, for azithromycin. There are
concerns about false-positive and false-negative reactions associated
with macrolide skin testing; therefore, graded challenge remains the
criterion standard. Furthermore, clarithromycin is not available in
intravenous form in the United States.
In vitro tests, such as BATs and lymphocyte transformation
tests, and detection of macrolide-specifi c IgE antibodies have
been used in several case reports, but they are yet to be stan-
dardized and are not commercially available.
Drug challenge tests remain the criterion standard to establish
or exclude macrolide hypersensitivity.
16,118,123
Drug challenge can
be performed for the suspected drug, a structurally related one, or
an alternative substance; however, the challenge must always be
carried out under strict medical surveillance and after a conscious
assessment of a risk-benefit analysis on a per-patient basis. It is best
performed as single-blinded, with increasing doses given every 30
minutes to achieve the maximum daily dose. Mori et al
122
used a
3-dose protocol, that is, 10%-20%-70% of the daily therapeutic
dose on day 1, followed by a full-dose administration on day 2. In
patients with a history of nonimmediate reactions, the challenge
was continued for 5 days at a therapeutic dose.
Other macrolides. Nonantibiotic macrolides have also been
involved in drug hypersensitivity reactions, ranging from allergic
contact dermatitis (ACD) due to topical application of tacroli-
mus in patients with atopic dermatitis, to generalized reactions
elicited by systemic administration. Saito et al
124
performed a
literature review on oral tacrolimuserelated drug hypersensitivity
reactions. Considering its immunosuppressive effects, it is not
surprising that most patch-test results (but not all) were negative
and most of these cases were diagnosed with a lymphocyte
stimulation test.
124,125
Severe reactions, namely, hypersensitivity
pneumonitis or DRESS, have been attributed (without drug
hypersensitivity workup) to drug-eluting stents involving zotar-
olimus and everolimus, respectively.
126,127
Management. Cross-reactivity among different macrolides has
not been extensively studied, but when it was tested, most patients
with a demonstrated hypersensitivity to a certain macrolide could
tolerate another with a different number of atoms in the lactone
ring.
80,116
Mori et al
122
reported double positivity in 2 patients
with clinical histories of anaphylaxis to clarithromycin (14-C) and
azithromycin (15-C). One of them had experienced anaphylaxis to
both drugs, whereas the other one had never taken clarithromycin.
Macrolide antibiotics are unlikely to cross-react with macro-
lide immunosuppressants, such as 23-C tacrolimus and 29-C
sirolimus. In a published case, cross-reactivity was suspected
between clarithromycin and tacrolimus, but for both drugs, the
assumption of drug hypersensitivity was based on clinical history
alone and no specific workup was performed.
128
Very few case reports of desensitization protocols to macro-
lides have been published (eg, to spiramycin in a pregnant
woman suffering from toxoplasmosis or to clarithromycin in 2
patients infected by Mycobacterium chelonae and Mycobacterium
avium, respectively) (Tables XV-XVII).
129-131
To our knowl-
edge, there are no reports to date for other commonly prescribed
macrolides, such as azithromycin and/or erythromycin.
Tetracyclines (by Stephanie Logsdon, MD)
General.
Tetracyclines are a broad-spectrum antibiotic class of
which 4 are available for systemic use in the United States:
tetracycline, demeclocycline, doxycycline, and minocycline. In
TABLE XII. Recommended concentrations of quinolones for skin
testing*
80,11 2 ,11 5
Quinolone
Concentration
prick (mg/mL)
Concentration
IDT (mg/mL) References
Moxifloxacin 1.6 Not performed Seitz et al,
112
2009
Tablet, 400 mg
suspended
in saline
Not performed Venturini et al,
115
2007
Ciprofloxacin 2 Not performed Seitz et al,
112
2009
0.02 0.02 Venturini et al,
115
2007
Levofloxacin 5 Not performed Seitz et al,
112
2009
5 0.05 Venturini et al,
115
2007
0.025 0.025 Empedrad et al,
80
2003
*Although these concentrations are currently recommended for skin testing, there is
controversy about nonirritating concentrations.
TABLE XIII. Recommended doses for drug provocation tests with
quinolones*
109,11 2
Quinolone
Doses administered
at intervals of 30 min
109
Doses administered
at intervals of 60 min
112
Moxifloxacin 5-50-100-100-150 25-50-100-200
Ciprofloxacin 5-50-100-150-200 50-125-250-500
Levofloxacin 5-50-100-150-200 50-125-250-500
*Increasing doses of the suspected fluoroquinolone were administered orally at in-
tervals of 30 min or 60 min until reaching the full dose or until symptoms of a drug
reaction occurred. Drug challenge protocols with less steps may also be considered
such as 1/10th of the dose followed by the full dose.
TABLE XIV. Recommended ciprofloxacin desensitization
procedure*
933
Time
(h) Route
Ciprofloxacin
concentration
(mg/mL)
Volume
given (mL)
Absolute
amount (mg)
Cumulative
total dose (mg)
0:00 IV 0.01 5.0 0.05 0.05
0:15 IV 0.1 1.0 0.1 0.15
0:30 IV 0.1 2.0 0.2 0.35
0:45 IV 0.1 4.0 0.4 0.75
1:00 IV 0.1 8.0 0.8 1.55
1:15 IV 1.0 1.6 1.6 3.15
1:30 IV 1.0 3.2 3.2 6.35
1:45 IV 1.0 6.4 6.4 12.75
2:00 IV 1.0 12.8 12.8 25.55
2:15 IV 10.0 2.5 25.0 50.55
2:30 IV 10.0 5.0 50.0 100.55
2:45 IV 10.0 10.0 100.0 200.55
3:00 Oral NA 250-mg tablet 450.55
IV, Intravenous; NA, not available.
*The patient should take the next oral dose of 500 mg that evening.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S28 BROYLES ET AL

addition, tigecycline is a glycylcycline, which is a new class of
antimicrobials with similar antimicrobial activity as the tetracy-
clines. Adverse reactions involving IgE-mediated hypersensitivity
are rare, and studies suggest that adverse reactions to minocycline
are more common than to doxycycline. Differences in side-chain
structures are hypothesized to be responsible.
132
Major symptoms. For all tetracyclines, most hypersensitivity
reactions are noneIgE-mediated, and include DRESS, papulosis,
lupus-like reactions, serum sicknesselike reactions, and photo-
sensitivity.
133-136
Nonetheless, IgE-mediated reactions do occur,
and anaphylaxis has been reported.
137-139
One report of drug fever
secondary to tigecycline use has also been published.
140
Rates of
cross-reactivity between tetracycline antibiotics are not established;
however, 1 report includes a patient with doxycycline hypersen-
sitivity who did exhibit cross-sensitization with minocycline.
141
Diagnosis. For patients with suspected IgE-mediated reactions,
skin testing is not standardized for any of the tetracycline antimi-
crobials, and positive and negative predictive values have not been
established. One case report has noted positive skin prick testing
result to full-strength tetracycline.
138
In addition, the minimum
nonirritating concentration for intradermal testing in control sub-
jects was 0.0002 mg/mL for minocycline and 0.001 mg/mL for
doxycycline. The following step-wise skin testing protocols have
been recommended: for minocycline, skin prick testing 0.2 mg/mL,
intradermal testing 0.00002 mg/mL, intradermal testing 0.0002
mg/mL. For doxycycline, skin prick testing 10 mg/mL, intradermal
testing 0.0001 mg/mL, intradermal testing 0.001 mg/mL.
142
Management. In patients with low clinical suspicion for
anaphylactic reactions and negative skin testing result, a graded drug
challenge may be considered.
13
In cases of high suspicion for
anaphylaxis or positive skin testing result where a tetracycline anti-
biotic is required, desensitization can be performed. There are no
published reports of effective rapid desensitization protocols for
these drugs, but some success has been seen with oral desensitization
to minocycline and doxycycline. A 14-step protocol was used for
minocycline, starting at 0.01 mg, followed by dose doubling at 30-
minute intervals until the cumulative goal was reached. Further-
more, an oral desensitization protocol for doxycycline (Table XVIII)
was successful, starting at 0.00001 mg and increasing in 10-fold
steps every 30 minutes until 1 mg was reached, and then increasing
by 2-fold increments until the goal dose was reached.
142
Successful desensitization to tigecycline using a 12-step pro-
tocol has also been recently reported.
142
Vancomycin (by Johnson Wong, MD)
General.
Vancomycin is a tricyclic glycopeptide antibiotic that
is used intravenously to treat various gram-positive cocci bacterial
infections including those caused by methicillin-resistant Staph-
ylococcus aureus and enterococcus, gram-positive cocci infections
among patients with
b
-lactam hypersensitivity, and gram-posi-
tive cocci infections in patients with renal failure. Oral vanco-
mycin is used to treat C difficile infections.
Major symptoms
“Red men syndrome”.
Red men syndrome (RMS) refers to
flushing and pruritus, which are commonly induced by the rate-
dependent direct mast cell degranulation (DMCD) effect of
vancomycin. In severe cases, hypotension, bronchospasm, and
urticaria may also accompany RMS. At a rate of 1 g/h or more,
10% to 80% of subjects developed RMS, whereas a much lower
incidence occurred when the rate was reduced to 1 g over 2
hours.
143
Histamine is often elevated early on, whereas tryptase
was not found elevated at 10 minutes. Treatment of choice of
TABLE XV. Desensitization protocol to spiramycin
130
Day Daily dose (IU)
1 300
600
900
1,200
3,000
9,000
12,000
Total: 33,000
2 30,000
60,000
90,000
120,000
150,000
180,000
Total: 630,000
3 300,000
600,000
900,000
1,200,000
3,000,000
Total: 6,000,000
4 3,000,000 thrice a day
Total: 9,000,000
5 3,000,000 four times a day
Total: 12,000,000
IU, International units.
TABLE XVI. Oral clarithromycin desensitization protocol
129
Step (15-min
intervals)
Clarithromycin
suspension
(mg/mL)
Volume
(mL)
Dose
(mg)
Cumulative
dose (mg)
1 0.05 0.1 0.005 0.0
2 0.05 0.2 0.01 0.0
3 0.05 0.4 0.02 0.0
4 0.05 1 0.05 0.1
5 0.05 2 0.1 0.2
6 0.05 4 0.2 0.4
7 0.5 0.8 0.4 0.8
8 0.5 1.6 0.8 1.6
9 0.5 3.2 1.6 3.2
10 0.5 6.4 3.2 6.4
11 5 1.2 6 12.4
12 5 2.4 12 24.4
13 5 4.8 24 48.4
14 50 1 50 98.4
15 50 2 100 198.4
16 50 4 200 398.4
17 50 8 400 798.4
18 50 10 500 1298.4
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S29

mild to moderate RMS is to slow the infusion rate to half the
previous infusion rate or 1 g per 2 hours or less, with antihis-
tamine pretreatment.
Anaphylactoid reactions/anaphylaxis/severe refractory
RMS.
This may occur in a small subgroup of patients. Although an
IgE-mediated mechanism is possible, most of these cases appeared to be
asevereformofDMCD.Vancomycin-specific IgE measurement has
not been reported in the literature. Skin testing has been proposed as a
surrogate for both DMCD sensitivity and possible IgE-mediated
sensitivity (see Skin Testing section below).
144-146
This highly sensitive
group can generally receive a rapid continuous intravenous protocol as a
method to induce temporary drug tolerance as described for vanco-
mycinaswellasotherdrugs.
147-150
Its advantage lies in the provision of
continuous small increments that avoid periodic substantial increases in
the rate of drug delivery. In several cases, one can reach a threshold level
only the first day and the dose need to be gradually increased over
several days. When possible, stopping concurrent narcotics or other
agents that also have DMCD property may often allow a difficult
desensitization to be successful.
Morbilliform rash/hematologic changes/DRESS.
Morbilliform rash is relatively common and often occurs in
isolation. It may also be accompanied by various combinations of
fever, hematologic changes, and/or end-organ dysfunction. The
hematologic changes commonly include eosinophilia and neu-
tropenia, and rarely, leukemoid reaction, lymphocytosis, throm-
bocytopenia, lymphadenopathy, and vasculitis. The end organs
that may be affected include kidney and liver. The combination of
drug reaction, eosinophilia, and systemic symptoms constitutes the
DRESS reaction, and a recent report indicated that HLA:A*32:01
is strongly associated with vancomycin-induced DRESS.
147
Another study suggested that circulating IFN-
g
vancomycin-
specific T cells may be isolated in such patients.
151
For patients
who are receiving multiple drugs and develop these reactions,
vancomycin should join
b
-lactam antibiotics high in the differ-
ential.
152
For patients with renal failure, vancomycin should be
considered as a culprit agent, because of its long half-life, even if the
reaction occurs weeks after the last dose. Management consists of
drug withdrawal and supportive treatment.
Linear IgA bullous diseases/TEN. Vancomycin is the
most commonly reported cause of the rare linear IgA bullous
diseases that may present as TEN-like reactions.
153
In addition,
TEN without identifying the mechanism has been reported.
Management is drug withdrawal and supportive treatment.
These reactions serve as absolute contraindication for re-
administration or desensitization.
Teicoplanin as alte rnative treatment. Teicoplanin is a
similar glycopeptide antibiotic that is available only outside the
United States. A major retrospective study and a prospective
study from the same institution over different time frames
showed that 12 of 117 (10%) and 14 of 24 (58%) patients,
respectively, who had a hypersensitivity reaction to vancomycin
also developed one to teicoplanin.
154,155
There was a high
recurrence rate and new occurrence rate for neutropenia,
leukopenia, and thrombocytopenia (10 of 14). Therefore, tei-
coplanin may not be an ideal alternative treatment option for
patients with vancomycin hypersensitivity.
Skin testing and implications. Skin testing has been
proposed as a surrogate for both DMCD sensitivity and possible
IgE-mediated sensitivity. Polk et al
146
performed titration of
vancomycin skin test reactivity as a function of vancomycin
concentration in a group of 12 healthy male volunteers. At
concentrations of more than or equal to 10
m
g/mL (0.02 mL
intradermally), all volunteers showed detectable wheal and flare.
The area of the flare, but not the wheal size, increased with
higher concentration, up to 40 to 100
m
g/mL (0.02 mL intra-
dermally) before reaching a plateau. Because these were healthy
volunteers without previous vancomycin exposure, the wheal-
and-flare responses were assumed to be due to DMCD at doses
of more than or equal to 10
m
g/mL (0.02 mL intradermally) and
not an IgE-mediated mechanism. The size of the flare at 25
m
g/
mL (0.02 mL intradermally) correlated poorly with the area of
flushing when the subjects were challenged with vancomycin
infusion. Case reports of patients with “anaphylactoid/anaphy-
laxis/pruritus” who developed wheal and flare at vancomycin
concentrations of 0.1 to 5
m
g/mL (0.02 mL intradermally) on
TABLE XVII. Oral clarithromycin desensitization protocol*
131
Dose no.
(15-min intervals)
Concentration
(mg/mL) Dose (mL) Dose (mg)
1 0.025 1.25 0.03
2 0.025 2.5 0.06
3 0.025 5 0.125
4 0.25 1 0.25
5 0.25 2 0.5
6 0.25 4 1
7 2.5 0.8 2
8 2.5 1.6 4
9 2.5 3.2 8
10 2.5 6.4 16
11 25 1.3 32
12 25 2.5 64
13 25 5 125
14 25 10 250
Cumulative dose 503
*Serial 10-fold dilutions of a clarithromycin suspension of 125 mg/5 mL (25 mg/mL)
were performed to make clarithromycin solutions at 2.5, 0.25, and 0.025 mg/mL.
TABLE XVIII. Doxycycline oral desensitization protocol*
Step Doxycycline (mg) Cumulative dose (mg)
1 0.00001 0.00001
2 0.0001 0.00011
3 0.001 0.00111
4 0.01 0.01111
5 0.1 0.11111
6 1 3.11111
7 2 3.11111
8 4 7.11111
9 8 15.11111
10 12 27.11111
11 25 52.11111
12 50 102.11111
Total time 360 min
*Steps with 30-min interva ls. Lowest dilution was based on 1:100 dilution of the
concentration that lead to positive skin testing result. Serial dilutions of doxycycline
suspension were prepared with purified water.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S30 BROYLES ET AL

skin testing were interpreted as representing IgE-mediated
sensitivity.
144,145
However, the cutaneous reactivity, even at 0.1
m
g/mL, may represent increased propensity for DMCD or
increased sensitivity to the mast cell release products (induced by
concurrent narcotic or due to intrinsic sensitivity of the pa-
tient).
150
Overall, the general consensus is that 50 mg/mL for
skin prick testing and 0.005 mg/mL for intradermal skin testing
are nonirritating concentrations (Table XIX).
Aminoglycosides (by Catherine Biggs, MD)
General.
Aminoglycosides are a broad-spectrum class of anti-
biotics that are structurally composed of hydrophilic sugars
possessing amine and hydroxyl functional groups.
156
Type IV
hypersensitivity reactions in the form of ACD are commonly
associated with aminoglycosides. The prevalence of ACD to
neomycin sulfate reported by the North American Contact
Dermatitis Group from 1985 to 2004 ranged from 7.2% to
13.1%.
157
Systemic contact dermatitis to aminoglycosides, as
well as severe type IV hypersensitivity reactions such as TEN and
DRESS syndrome, have also been described.
158-160
IgE-mediated
reactions to aminoglycosides are uncommon but may occur after
exposure to topical, inhaled, and systemic preparations.
161-163
Major symptoms of hypersensitivity. IgE-mediated re-
actions described in the literature include urticarial reactions in
response to both intravenous and inhaled tobramycin, as well as
anaphylaxis to topical and systemic aminoglycosides.
161-163
ACD
may present as erythematous/pruritic/edematous papules, pla-
ques, and/or vesicles at the site of contact.
157
TEN caused by
streptomycin has been associated with high fever, vomiting, and
diffuse erythema, followed by extensive bullae formation and
skin denudation.
159
Features of DRESS syndrome include a
maculopapular and edematous skin rash, facial edema, and fever;
laboratory studies may demonstrate eosinophilia, atypical
lymphocytosis, abnormal liver enzymes, and coagulopathy.
158
Diagnosis. IgE-mediated reactions are diagnosed on the basis
of clinical features in keeping with an immediate hypersensitivity
reaction, and have been confirmed in the literature using skin
testing. Systemic reactions to epicutaneous and intradermal
testing have been described in patients with a history of
anaphylaxis to topical and systemic aminoglycosides, respec-
tively.
161,164,165
Skin testing should therefore be approached
with caution, and one may consider beginning with a 10-fold
dilution for the first intradermal dose in patients with a history of
anaphylaxis to an aminoglycoside (Table XX).
The diagnosis of type IV hypersensitivity reactions may be
supported diagnostically by patch testing to the aminoglycoside
in question. Significant cross-reactivity among aminoglycosides
has been reported in ACD.
157
Management. Avoidance of the offending agent is recom-
mended for patients with a history of type IV hypersensitivity
reactions. In the setting of negative skin testing result and low
clinical suspicion for an IgE-mediated allergy, a graded challenge
can be performed. Rapid desensitization is recommended for
patients with an IgE-mediated allergy to an aminoglycoside who
require the medication when no suitable alternative antibiotic
exists.
167
Table XXI presents an example of a desensitization
protocol for tobramycin.
Clindamycin (by Jocelyn R. Farmer, MD)
General.
Clindamycin, a semisynthetic derivative of linco-
mycin, is a bacteriostatic agent that inhibits protein synthesis. It
has been on the market since 1968 and is generally well tolerated.
Common adverse reactions include a metallic taste in the mouth,
transient elevation of transaminases, and a propensity for C
difficile infection, with an increased risk for diarrhea (10%-23%)
and pseudomembranous colitis (2%).
168
Major symptoms of hypersensitivity. Immediate hy-
persensitivity to clindamycin is rare, though clindamycin-
induced anaphylaxis has been reported.
169-171
Delayed hyper-
sensitivity to clindamycin is much more common, initially esti-
mated at 10% in small clinical studies from the 1970s, but
revised to be less than 1% in a large retrospective chart review of
clindamycin administration at a single US center from 1995 to
1997.
172-174
Delayed maculopapular exanthems are predomi-
nantly seen; however, SJS, DRESS, TEN, sweet syndrome, and
AGEP have all been reported in the literature.
175-183
TABLE XIX. Vancomycin rapid intravenous desensitization
protocol*†
Time
(h:min)
Vancomycin
concentration
(mg/mL)
Fluid infusion
rate (mL/h)
Vancomycin
infusion rate
(mg/h)
Cumulative
dose (mg)
0:00 0.0001z 60.0 0.0060 0
0:15 0.001 20.0 0.020 0.0015
0:30 0.001x 60.0 0.060 0.0065
0:45 0.01 20.0 0.20 0.022
1:00 0.01 60.0 0.60 0.072
1:15 0.1 20.0 2.0 0.22
1:30 0.1 60.0 6.0 0.77
1:45 1.0 20.0 20 2.2
2:00 1.0 60.0 60 7.7
2:15 10 12.5 125 22
2:30k{# 10 25.0 250 54
*Adapted from the original protocol of Wong et al.
150
†H
1
antihistamine pretreatment.
zTypical starting concentration for patients with severe systemic reactions to pre-
vious vancomycin infusions.
xTypical starting concentration for patients with moderate systemic reactions to
previous vancomycin infusions.
kContinue at this infusion rate for the remainder of the dosage.
{Minimize concurrent narcotic and other direct mast degranulators if possible.
#May need to stay just below a threshold vancomycin infusion rate the first day and
advance as tolerated.
TABLE XX. Immediate hypersensitivity testing recommended for
aminoglycosides
80,162,166
Aminoglycoside SPT dilutions (mg/mL) IDT dilutions (mg/mL)
Gentamycin
(preservative free)
40 0.04*
0.4
4
Tobramycin 40 0.04*
0.4
4
*Optional step that is recommended for patients with a history of anaphylaxis to an
aminoglycoside.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S31

Clindamycin-induced cytopenias have also been described,
including anemia, neutropenia, and thrombocytopenia. The
underlying mechanism is unclear, though direct myelosup-
pression at the level of the hematopoietic stem cell has been
shown.
184
Diagnosis. Immediate hypersensitivity skin testing to clinda-
mycin has been studied. Skin prick at 150 mg/mL and intra-
dermal injection at 15 mg/mL have been identified as
nonirritating concentrations.
80,185
However, skin testing for
immediate hypersensitivity to clindamycin was shown to be
inferior to direct drug challenge in a study of 31 patients with a
history of adverse clindamycin reaction, where 0 of 31 patients
demonstrated a positive skin test result but 10 of 31 patients
(32%) reacted during an oral challenge with 150 mg clindamy-
cin.
185
The utility of immediate hypersensitivity skin testing to
clindamycin in routine clinical practice has been questioned. In
contrast, delayed hypersensitivity patch testing to clindamycin
has been more promising. In patients with a predominant history
of delayed maculopapular exanthems, patch testing via clinda-
mycin 150-mg tablet pulverized and diluted in 1 mL saline or via
pure clindamycin diluted to 10% in petrolatum resulted in
positive reactions in 15% to 30% of patients, respectively.
186,187
Therefore, clindamycin patch testing may be useful in the
context of a convincing history of delayed hypersensitivity.
Management. Most adverse reactions to clindamycin are
mild and the drug can be continued safely. In cases of severe
DHRs to clindamycin (eg, SJS, DRESS, TEN, and AGEP), the
drug should be empirically avoided and there are no suitable
alternatives. For nonsevere DHRs to clindamycin (eg, isolated
maculopapular exanthem), a single case of oral desensitization
has been described.
188
The DHR to clindamycin was confirmed
on repeat oral challenge. The starting dose for the desensitization
was clindamycin 20 mg every 8 hours, with dose escalation over
7 days as given in Table XXII. The patient was without adverse
reaction at the 13-month follow-up, demonstrating proof of
principle for clindamycin desensitization in the treatment of
delayed drug hypersensitivity. More recently, clindamycin
desensitization in the treatment of immediate drug hypersensi-
tivity was reported in the literature in a single pediatric case using
a rapid 9-step oral clindamycin desensitization protocol over 4
hours to a cumulative dose of 11 mg/kg per dose (300 mg/dose),
which was then continued every 8 hours for the duration of the
antimicrobial course.
189
Linezolid (by Jocelyn R. Farmer, MD)
General.
Linezolid is an oxazolidinone that works by inhibit-
ing the initiation of bacterial protein synthesis. It was first
introduced for clinical use in the United States in 2000 and is
generally well tolerated. Among the known adverse reactions,
linezolid-induced cytopenias are best described and occur more
frequently with long-term therapy (>14 days), increased daily
dose (22 mg/kg), high serum concentration, and comorbidities
including renal insufficiency.
190-193
Prevalence rates vary widely
by study design but have been estimated at 15% to 50% for
thrombocytopenia, 4.2% to 16% for anemia, and 2.2% to 4.5%
for leukopenia.
194-196
The underlying mechanism is felt to be
myelosuppression secondary to off-target inhibition of host
protein synthesis in the bone marrow, which aligns with the
dose-dependent and reversible nature of linezolid-induced cyto-
penias and the fact that linezolid-dependent antiplatelet anti-
bodies have not been described.
197
Less frequent adverse
reactions to linezolid include lactic acidosis, which typically oc-
curs with long-term therapy (>6 weeks) and has been attributed
to the underlying mitochondrial inhibitory mechanism of the
drug.
198
In addition, there has been a case report of peripheral
and optic neuropathy.
199-201
Major symptoms of hypersensitivity. Immediate hy-
persensitivity to linezolid is rare. In a review of 828 linezolid
treatment courses from 1997 to 2000, treatment-limiting
dermatologic events including rash and pruritus occurred in
1.7% of cases, and only 2 patients had anaphylactic-type re-
actions.
194
Subsequently, a case of immediate urticaria and
angioedema to linezolid and 2 more detailed descriptions of
suspected linezolid anaphylaxis were reported in the litera-
ture.
202-204
Delayed hypersensitivity to linezolid is also infre-
quent and can include acute interstitial nephritis and/or
TABLE XXI. Example of a desensitization protocol for intravenous
tobramycin*
167
Tobramycin Full therapeutic dose [ 100 mg IV q8h
Tobramycin
solutions†
1. 1 mg/200 mL NS
(final concentration ¼ 0.005 mg/mL)
2. 10 mg/200 mL NS
(final concentration ¼ 0.050 mg/mL)
3. 99 mg/200 mL NS
(final concentration ¼ 0.495 mg/mL)
Step Solution
Rate
(mL/h)
Time
(min)
Administered
dose (mg)
Cumulative
dose (mg)
1 1 2.5 15 0.0031 0.0031
2 1 5 15 0.0063 0.0094
3 1 10 15 0.0125 0.0219
4 1 20 15 0.0250 0.0469
5 2 5 15 0.0625 0.1094
6 2 10 15 0.1250 0.2344
7 2 20 15 0.2500 0.4844
8 2 40 15 0.5000 0.9844
9 3 10 15 1.2377 2.2221
10 3 20 15 2.4754 4.6975
11 3 40 15 4.9508 9.6482
12 3 80 136.875 90.3518 100.0000
IV, Intravenous; NS, normal saline; q8h, every 8 h.
*Dosage will vary on the basis of indication and patient weight.
†The total volume and dose dispensed of the tobramycin solutions are more than the
final dose given to patient because the initial solutions are not completely infused.
TABLE XXII. Protocol for clindamycin desensitization for the
management of delayed hypersensitivity
188
Day Oral clindamycin dose (mg q8h)
120
240
380
4 150
5 300
6 600
7 600 (q6h)
q6h, Every 6 h; q8h, every 8 h.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S32 BROYLES ET AL
DRESS.
205-208
In a recent systematic chart review of 824 out-
patients receiving monitored antibiotic therapy, linezolid was
significantly associated with increased risk for peripheral eosin-
ophilia but not end-organ damage including nephritis and
DRESS.75 Together, these data suggest a known but small risk
for severe DHRs after linezolid therapy.
Diagnosis. To date, no standardized immediate or delayed
hypersensitivity skin testing protocols for linezolid have been
published. Diagnosis is therefore limited to direct oral or intra-
venous challenge in cases in which it is clinically safe to do so.
Management. Treatment-limiting adverse reactions to line-
zolid include gastrointestinal complaints (with oral preparations),
cytopenias, rash, and angioedema.
194,195
In cases of severe DHRs
to linezolid (eg, acute interstitial nephritis or DRESS), the drug
should be empirically avoided. Alternatively, for immediate hy-
persensitivity reactions, linezolid desensitization has been
described.
202,203
Given that the drug has an oral bioavailability of
approximately 100% in healthy volunteers, the first protocol
used oral desensitization to linezolid. The patient tolerated 14
sequential dilutions in the absence of breakthrough symp-
toms.
203
More recently, intravenous linezolid desensitization for
immediate hypersensitivity was accomplished as presented in
Table XXIII. The patient had no breakthrough symptoms and
subsequently made a transition to oral linezolid 600 mg every 12
hours to complete a 2-week course.
Nitrofurantoin (by Jocelyn R. Farmer, MD)
General.
Nitrofurantoin is a broad-spectrum antibiotic that
was first approved as clinical treatment for urinary tract infections
in 1953. Intracellular nitroreductase produces the active drug
metabolite, which functions to inhibit bacterial DNA and RNA
synthesis.
209
Adverse reactions to nitrofurantoin are infrequent
(5%-16%) and generally mild, reversible, and predominantly
gastrointestinal (nausea and abdominal discomfort).
210
Major symptoms of hypersensitivity. Immediate hy-
persensitivity reactions to nitrofurantoin are rare. Nitrofurantoin-
induced anaphylaxis has been reported, and acute pulmonary
reactions with symptoms consisting of shortness of breath,
cough, fever, and peripheral eosinophilia within days to weeks of
drug initiation have been reported at a rate of 1 in 5000 first
administrations.
211-214
Lung pathology can demonstrate vascu-
litis, mild interstitial inflammation, eosinophils, and reactive type
II pneumocytes.
215
In general, with drug discontinuation, pa-
tients have prompt recovery, and nitrofurantoin-induced acute
pulmonary reactions have an overall mortality rate of only
0.5%.
216
Delayed hypersensitivity to nitrofurantoin can include
pulmonary fibrosis, hepatotoxicity, erythema multiforme, ery-
thema nodosum, agranulocytosis, megaloblastic anemia, and
optic neuritis.
210
However, these adverse reactions are rare, with
incidence rates per nitrofurantoin course previously calculated at
0.001% for all pulmonary reactions combined, 0.0003% for
hepatotoxicity, 0.0004% for hematologic events, and 0.0007%
for neurologic complications.
217
Nitrofurantoin-induced pul-
monary fi brosis and hepatotoxicity have been associated with
systemic autoantibody production and lymphocytic infiltrates to
the end organ with subsequent risk for fibrosis.
215,218
An un-
derlying mechanism of direct drug injury (via reactive oxygen
species) and indirect drug injury (via induced cellular hyper-
sensitivity) has been described.
219,220
In general, patients have
pronounced recovery with drug cessation, though occasionally
steroid therapy is also required.
215,218
Diagnosis. To date, no standardized immediate or delayed
hypersensitivity skin testing protocols for nitrofurantoin have
been published despite a few case reports.
214,221
Diagnosis is
therefore limited to direct oral challenge in cases in which it is
clinically safe to do so.
Management. Most adverse reactions (eg, nausea and
abdominal discomfort) are mild and the drug can be continued
safely. However, in rare cases of severe nitrofurantoin-induced
immediate hypersensitivity reactions (eg, anaphylaxis and acute
pulmonary reactions), or DHRs (eg, pulmonary fibrosis and
hepatitis), nitrofurantoin should be discontinued immediately
and empirically avoided. Case reports of drug desensitization
have not been described.
Antituberculous drugs (by Stephan ie Logsdon, MD,
and Josefina Cernadas, MD)
Introduction.
Tuberculosis (TB), an infection caused by
Mycobacterium tuberculosis, is a curable infectious disease, prev-
alent worldwide, and potentially fatal if proper treatment is not
instituted. It remains a major cause of global mortality and
morbidity. Most of the 2 billion people estimated to be infected
with M tuberculosis have asymptomatic infection, termed latent
TB infection.
222-224
Similar to other Mycobacterial infections, treatment of TB
requires simultaneous administration of multiple drugs. In the
absence of bacterial resistance, treatment usually consists of
isoniazid (INH), rifampicin, pyrazinamide (PZA), ethambutol
(EMB), and streptomycin for 6 months.
Although most treatment courses progress with minor side
effects, ADRs can occur. No consensus has been reached con-
cerning the overall incidence of ADRs to these drugs. Different
studies report an incidence of 5.5% to 57.8% according to
different populations and ADR definitions.
225-230
Adverse effects
or drug interactions can make it necessary to modify or discon-
tinue treatment.
A major ADR to any of the anti-TB drugs, which implicates
the discontinuation of that drug, can have severe implications.
Alternative agents may lead to greater toxicity and are often less
effective, frequently requiring longer treatment courses. As a
result, the risk of treatment failure and relapse is higher in these
cases. In these patients, management is further complicated by
the difficult task of identifying the culprit agent for the reaction
when they are on a typical multidrug regimen. In patients with
SCARs, there are reports of further challenge with selected drugs
because of the limited availability of treatment; however, as with
any other SCAR, avoidance is typically recommended.
231
Rifampin (Rifampicin). Rifampin remains a common ther-
apy for latent and active TB, and is also used for infections with
aerobic gram-negative organisms including the asymptomatic
Neisseria meningitidis carrier state. It is rarely implicated in hy-
persensitivity reactions, ranging from pruritic skin eruptions to
anaphylaxis, despite its frequent use, but is associated with
multiple side effects.
Side effects of rifampin include an influenza-like syndrome,
hepatotoxicity, skin exanthems, and induction of SMZ-TMP,
which may cause significant drug-drug interactions.
232
The most
commonly reported ADR is the influenza-like syndrome, which
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S33

consists of fever, chills, headaches, myalgia, and rash. Published
reports have also described rifampin hypersensitivity reactions in
both adult and pediatric populations, ranging from pruritic skin
eruptions to anaphylaxis.
233
Diagnosis of rifampin hypersensitivity can be difficult, because
many reactions are likely not IgE-mediated. Skin testing can be
helpful in determining the likelihood of reaction, though nega-
tive skin test reactions do not indicate lack of sensitivity. Skin
testing should be performed on the basis of published protocols
in which the highest intradermal nonirritating drug concentra-
tion in nonallergic control subjects was 0.002 mg/mL.
234
Similar
nonirritating intradermal concentrations have been reported in
the literature for evaluation of rifampin hypersensitivity
.
235
Rifampin is a critical component of anti-TB therapy and often
alternative drugs are not available. Therefore, in cases of hyper-
sensitivity to rifampin, desensitization to the medication is often
required. Recommended dosing of rifampin for TB is 600 mg
daily, but maintaining a desensitized state with this dose can be
problematic secondary to the drug’s short half-life of 3
hours.
235,236
Despite this, various techniques for desensitization
have been published, from rapid protocols to those of 7 or more
days’ duration.
235,237
Three pediatric case reports have been
published.
238-240
Although uncommon, adverse reaction during
rapid desensitization to rifampin may occur. In 1 previously
published report, a pediatric patient developed a reaction during
the protocol despite premedication, whereas the other 2 patients
tolerated rapid desensitization without premedication. Another
report described an adult patient who developed anaphylaxis
during an oral desensitization procedure after positive skin
testing result.
241
A new, alternating-dose rapid oral desensitization protocol for
rifampin was completed in a pediatric patient to achieve appro-
priate serum concentrations while safely maintaining the desen-
sitized state.
240
The protocol used a regimen of 600 mg in the
morning followed by 300 mg rifampin in the evening. This
dosing was chosen because of the short half-life of rifampin, to
maintain desensitization. For oral rifampin, the 13-step rapid
oral desensitization protocol outlined in Table XXIV is effective
and safe. Strict adherence to an every-12-hour dosing schedule is
required to safely maintain the desensitized state.
Pyrazinamide. PZA, a synthetic pyrazine analogue of nico-
tinamide, is one of the most effective anti-TB drugs and is
generally well tolerated. Like any other drug it may cause adverse
reactions. These reactions are mainly toxic and may affect several
organs: liver (cytolysis), joints (arthralgia), and the gastrointes-
tinal system with nausea, vomiting, diarrhea, and abdominal
pain. This drug may also induce more severe reactions such as
nephrotoxicity, but the most severe one is hepatotoxicity ranging
to fulminant hepatitis. Also, the drug most likely responsible for
the occurrence of hepatitis during therapy for active TB is PZA.
Cutaneous manifestations are the most common reactions to
PZA, though true allergic reactions are rare
.
242-244
It can also be
associated with early onset of maculopapular rash with pruritus,
sometimes in association with dyspnea (possibly due to bron-
chospasm) and abdominal pain, suggesting anaphylactic/
anaphylactoid reaction. The mechanisms underlying these re-
actions are undetermined.
225,245
Among anti-TB medications, the incidence of serious side
effects, defined as those requiring a documented change in
therapy or hospitalization, is highest with PZA. These serious
side effects are associated with female sex, older age, Asian origin,
and HIV infection.
225
Hypersensitivity reactions should be suspected if an immedi-
ate skin rash develops at the initiation of PZA treatment. If the
degree of skin involvement is not severe, sequential reintro-
duction of the drugs first at low, then at full dosage may be
attempted.
Bavbek et al
246
described an allergic reaction in a patient who
had positive skin prick test (SPT) result to PZA, with negative
results in 10 controls, and a positive oral challenge test result.
246
The SPTs were performed with PZA tablets at a concentration of
500 mg/mL along with a positive histamine control and a
negative saline control 1 week after the initial reaction. The PZA
tablets were smashed in a mortar and diluted with 1 mL of 0.9%
NaCI. A positive reaction was seen only with PZA, producing a
TABLE XXIII. Protocol for linezolid desensitization for the management of immediate hypersensitivity
202
Step Bag (no.)
Bag
(mg/mL in D5W) Time
Rate
(mL/h)
Infusion
duration (min)
Monitoring
duration (min)
Volume
infused (mL) Dose (mg)
Cumulative
dose (mg)
1 1 0.02 0:00 18.0 5 10 1.50 0.03 0.03
2 1 0.02 0:15 42.0 5 10 3.50 0.07 0.10
3 2 0.20 0:30 9.0 5 10 0.75 0.15 0.25
4 2 0.20 0:45 18.0 5 10 1.50 0.30 0.55
5 2 0.20 1:00 36.0 5 10 3.00 0.60 1.15
6 2 0.20 1:15 72.0 5 10 6.00 1.20 2.35
7 3 2.00 1:30 13.8 5 10 1.20 2.30 4.65
8 3 2.00 1:45 28.2 5 10 2.30 4.70 9.35
9 3 2.00 2:00 56.4 5 10 4.70 9.40 18.75
10 3 2.00 2:15 112.5 5 10 9.40 18.75 37.50
11 3 2.00 2:30 225.0 5 10 18.80 37.50 75.00
12 3 2.00 2:45 450.0 5 10 37.50 75.00 150.00
13 3 2.00 3:00 300.0 15 10 75.00 150.00 300.00
14 3 2.00 3:25 600.0 15 30 150.00 300.00 600.00
Total time 4:10
DSW, Dextrose 5% in water.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S34 BROYLES ET AL

wheal of 3 mm surrounded by erythema of 5 mm after 15 mi-
nutes.
246
The positive SPT result suggested an IgE-mediated
mechanism, although in vitro measurement of PZA-specific IgE
in the circulation was not conducted.
In cases of vital indication of this drug, when no alternative
treatment is available, drug desensitization is an option
(Table XXV).
225
Isoniazid. INH is a bactericidal antibiotic against M tubercu-
losis. It acts by inhibiting mycolic acid biosynthesis. INH also
disrupts DNA, lipid, carbohydrate, and nicotinamide adenine
dinucleotide synthesis, and/or metabolism. The incidence of
adverse reactions to INH in more than 2000 patients has been
estimated to be 5.4%.
225,247
Although the incidence of adverse reactions to INH is low, the
best described are hepatic, neurologic, skin reactions, and fe-
ver.
248-253
Skin eruptions are rare, mild, and often transient, and
usually consist of morbilliform and maculopapular rashes, urti-
caria, and pruritus.
254-256
Cutaneous reactions in patients
receiving therapy are often difficult to diagnose because several
drugs are frequently taken simultaneously. The liver is the most
commonly affected organ and up to 20% of patients experience
mild liver injury, which is usually subclinical and self-limited.
257
A less common form of reaction to INH is hepatitis, a more
serious form of liver injury that can be fatal. The mechanism of
liver damage is not completely understood, though it seems
related to the direct toxicity of the drug.
The diagnosis is based on the regression of symptoms with
discontinuation of the drug and the reproducibility with rein-
troduction. The reintroduction of antibiotics separately is
necessary to identify the culprit(s). This approach to diagnosis
cannot be done in the presence of severe reactions such as
exfoliative dermatitis and hepatotoxicity. These are absolute
contraindications for challenge.
The rare cases of type I IgE-mediated allergic reaction require
study with skin testing, with injectable forms for prick and in-
tradermal tests.
258
Rodrigues Carvalho et al
259
performed skin testing in a case of
a generalized maculopapular rash, fever, and diarrhea after
treatment with several anti-TB drugs. Prick test (PT) with INH
was performed with an undiluted solution of 100 mg/mL and
IDT with a 10 mg/mL concentration.
Several protocols have been published regarding the desensi-
tization to INH based on adapted protocols from desensitization
to penicillin (Table XXVI).
239,259-261
Ethambutol. EMB is a bacteriostatic antimycobacterial drug
most commonly used in combination with other drugs in the
treatment of TB. The mechanism of action is not completely
known. There is evidence that the drug exerts its bacteriostatic
activity by inhibiting arabinosyltransferase, an enzyme that po-
lymerizes arabinose into arabinan and then arabinogalactan, a
mycobacterial cell wall constituent.
The dose of EMB is dependent on body weight, frequency of
administration, and indication. It is generally well tolerated. Side
effects are usually dose-related and more common when doses
exceed 15 mg/kg.
The main side effect of EMB is ocular toxicity due to optic
neuritis. Intermittent dosing may decrease the risk of ocular
toxicity, as confirmed by Griffith et al
262
in a study of 229 pa-
tients treated with EMB for pulmonary M avium complex
disease.
The combination of EMB with PZA for the treatment of
latent TB in patients exposed to multidrug-resistant strains has
been associated with a high incidence of hepatotoxicity or
gastrointestinal intolerance, leading to discontinuation of therapy
(w60% of treated subjects in 1 report).
263
Hypersensitivity reactions to EMB such as rash and drug fever
have been reported in 0.5% and 0.3% of patients, respec-
tively.
264
Other reactions, such as dermatosis-like pigmentation,
lichenoid eruptions, pulmonary infiltrates, and TEN, have also
been described.
265-268
In cases of severe reactions such as exfoliative dermatitis, oral
challenge tests are not recommended. Skin and serological tests
are usually unreliable, with single reports of positive patch-test
TABLE XXIV. Rifampin oral desensitization protocol*
240
Solution Volume Concentration
Solution A 10 mL 0.001 mg/mL
Solution B 10 mL 0.01 mg/mL
Solution C 30 mL 0.1 mg/mL
Solution D 10 mL 6 mg/mL
Solution E 15 mL 60 mg/mL
Step no.
Dose
(mg)
Solution
no.
Volume
(mL)
Time
(min)
Cumulative
dose (mg)
1 0.0002 A 0.2 30 0.0002
2 0.002 B 0.2 30 0.002
3 0.02 B 2 30 0.02
4 0.2 C 2 30 0.2
5 2 D 0.3 30 2.2
6 4 D 0.7 30 6.2
7 8 D 1.3 30 14.2
8 16 D 2.7 30 30.2
9 30 E 0.5 30 60.2
10 50 E 0.8 30 110.2
11 100 E 1.7 30 210.2
12 150 E 2.5 30 360.2
13 250 E 4.2 30 610.2
*Full therapeutic dose administered 12 h after initiation of step 13.
TABLE XXV. Oral desensitization protocol with PZA
259
Time (min) Dose (mg) Cumulative dose (mg) Reactions
Day 1
0 6.25 6.25
30 12.5 18.75
60 25 43.75
90 50 93.75
150 75 168.75
210 125 239.75
270 250 543.75
Day 2
0 500 500
30 1000 1500
Day 3
0 750
60 750 Total
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S35

results and positive lymphocyte stimulation test result to
EMB.
254
Skin prick and intradermal tests can be performed according
to European Academy of Allergy and Clinical Immunology
(EAACI) guidelines.
269
Immediate and late readings of IDT
performed with sterile solutions of 1 mg/mL and 10 mg/mL are
necessary because hypersensitivity reactions are mostly nonIgE-
mediated. Immediate reading can be negative with late positive
intradermal readings.
270
The decision to desensitize should be
made in conjunction with the infectious disease specialist to
determine the benefitoffirst-line therapy over alternatives, the
duration of treatment, and the goals of therapy.
Drug desensitization should not be attempted in cases of se-
vere skin reactions or those involving the mouth or mucous
membranes (eg, exfoliative dermatitis and SJS). Tables XXVII,
XXVIII, and XXIX summarize one institution’s protocols that
were used for several patients from the infectious disease
department who had cutaneous hypersensitivity reactions to
EMB.
Rodrigues Carvalho et al
259
described successful use of a
modified 1-day temporary induction of tolerance protocol in a
patient with reactions to several anti-TB drugs. The protocol
consisted of a 2-fold increase in drug dose every 45 minutes until
the desired total daily dose was reached.
Another protocol was used in a clinically non-IgE hypersen-
sitivity reaction to EMB in which the previously described pro-
tocol was ineffective.
44
The patient had facial erythema,
angioedema of the neck and upper limbs followed by a pruritic
scaling maculopapular exanthem involving the neck, trunk, and
upper and lower limbs, associated with mild dyspnea with no
hemodynamic change 2 months before finishing treatment with
EMB. Symptoms resolved after stopping the drug and reap-
peared after reintroduction. Skin tests were performed using
solutions of 1 mg/mL and 10 mg/mL prepared from diluting a
crushed 400-mg EMB tablet.
271
Immediate reading result was
negative, but a positive intradermal reaction was present at 6
hours, with resolution at 72 hours. Because of the clinical pre-
sentation, positive delayed skin testing result, and reproducibility
of the reaction on reexposure, a hypersensitivity reaction to EMB
was diagnosed. The applied protocol was designed on the basis of
the 12-step protocol by Castells et al (Table XXX), using slower
dose increments and premedication with 25 mg of hydroxyzine
and 40 mg of oral prednisone.
271,272
Streptomycin. Streptomycin is in the aminoglycoside class of
medications and can be used to treat TB in combination with
other medications. It works by blocking the ability of 30S ri-
bosomal subunits to make proteins that result in bacterial death.
Common side effects include dizziness, vomiting, numbness of
the face, fever, and rash.
159
Most ADRs due to intramuscular injection of streptomycin
are gastrointestinal problems (38.09%), followed by skin re-
actions (30.48%) and hepatotoxicity (14.28%).
158
Streptomycin is vestibulotoxic, ototoxic, and nephrotoxic.
Nephrotoxicity can potentially interfere with the diagnosis of
kidney malfunction. The most concerning side effects, as with
other aminoglycosides, are nephrotoxicity and ototoxicity.
Ototoxicity and nephrotoxicity are more likely to be found when
therapy is continued for more than 5 days and at higher doses. In
very high doses, streptomycin can also produce a curare-like ef-
fect with neuromuscular blockade that results in respiratory
TABLE XXVI. Rapid oral tolerance induction protocol to
isoniazid*
259
Time (min) Dose (mg) Cumulative dose (mg)
0 0.050 0.050
20 0.10 0.15
40 0.25 0.40
60 0.50 0.90
80 1.00 1.90
100 2.00 2.90
120 4.10 8.00
140 8.20 16.20
160 16.30 32.50
180 30.60 63.10
200 50.30 113.40
340 100 213.40
480 (8 h) 150.00 363.40 (total daily dose)
*Serial dilutions of the 50 mg/5 mL suspension were prepared using purified water.
TABLE XXVII. General protocol for oral desensitization to EMB in
adults*
Time from start (h:min) EMB 1 mg/mL
0:00 0.1
00:45 0.5
01:30 1
02:15 2
03:00 4
03:45 8
04:30 16
05:15 32
06:00 50
06:45 100
07:30 200
11:00 400
Next day, 06:30 400 thrice a day
*Serial dilutions of the 50 mg/5 mL suspension were prepared using purified water.
TABLE XXVIII. Rapid oral tolerance induction to EMB*
259
Time (min) Dose (mg) Cumulative dose (mg)
0 0.10 0.10
45 0.50 0.60
90 1.00 1.60
135 2.00 3.60
180 4.00 7.60
225 8.00 15.60
270 16.00 31.60
315 32.00 63.60
360 50.00 113.60
405 100.00 213.60
450 200.00 413.60
495 400.00 813.60
660 (11 h) 400.00 1213.60 (total daily dose)
*Serial dilutions of the 50 mg/5 mL suspension were prepared using purified water.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S36 BROYLES ET AL

paralysis. It is not recommended in patients with myasthenia
gravis.
273
In general, streptomycin has little allergenic potential, and
hypersensitivity reactions are rare. However, skin rashes, eosin-
ophilia, fever, blood dyscrasias, angioedema, exfoliative derma-
titis, stomatitis, and anaphylactic shock have been reported.
274
Sanchez-Borges et al
275
report that positive skin test results
have been observed with streptomycin. However, a cautious
approach must be taken when evaluating anaphylactic reactions
to streptomycin, because systemic reactions have been observed
after skin prick testing. The starting concentrations suggested for
SPTs range from 0.1 to 1 mg/mL, gradually reaching the con-
centration of 20 mg/mL if tolerated. If SPT results are negative,
intradermal testing can be performed and nonirritating concen-
trations of 4 mg/mL for intradermal testing have been established
for gentamicin and tobramycin. There is no evidence of positive
serum IgE to aminoglycosides. Patch tests with reading at 72 and
96 hours are recommended for the diagnosis of nonimmediate
reactions. The concentration is 1% in petrolatum for
streptomycin.
275
In patients with streptomycin hypersensitivity, avoidance of
the antibiotic is recommended. There are 2 reports of desensi-
tization to streptomycin, one describing a 3-hour protocol with
streptomycin beginning with 1 mg administered intravenously
and the other consisting of streptomycin given intramuscularly,
10 mg, on the first day and doubled every day until it reached
800 mg, then 1.0 g, twice weekly.
276,277
Patients reached 1.0 g of
streptomycin on the ninth day.
Metronidazole (by Sara Barmettler, MD)
General.
Metronidazole is a 5-nitroimidazol compound that is
active against a wide array of anaerobes, protozoa, and micro-
aerophilic bacteria. Metronidazole is the treatment of choice for
most anaerobic infections, including mild to moderate C difficile
infections.
278
The 5-nitroimidazole drugs (including metroni-
dazole or tinidazole) are the only class of drugs that provide
curative therapy for trichomoniasis and thus, given the low ef-
ficacy of any drug other than the 5-nitroimidazole drugs, the
Centers for Disease Control and Prevention guidelines recom-
mend that patients with trichomoniasis who have experienced an
IgE-mediated allergy to metronidazole and/or tinidazole be
referred for desensitization rather than using an alternative class
of drugs.
279
Major symptoms. A number of nonallergic adverse effects of
metronidazole have been described, including gastrointestinal
symptoms, nervous system effects, genitourinary effects, and
disulfiram-like reactions.
278
Hypersensitivity reactions caused by
metronidazole appear to be rare, and case reports have been
infrequently described in the literature. Several types of reactions
have been reported, including immediate reactions and
anaphylaxis, delayed reactions, fixed drug eruption, serum
sicknesselike reaction, and SJS/TEN.
280-285
Diagnosis. Skin testing for metronidazole hypersensitivity has
been described.
280,286
Skin prick testing was performed at a
concentration of 125 mg/mL. Using this concentration, a patient
tested in 1 study had previously suspected anaphylaxis to
metronidazole and was positive on skin prick testing on 2
different occasions.
280
This case report also found skin prick
testing result with metronidazole to be negative in 10 control
patients.
280
In another case series, 4 patients with a history of
cutaneous pruritic exanthemas were skin tested with prick testing
at a concentration of 125 mg/mL of metronidazole followed by
IDT with metronidazole at 0.5%, 5%, and 10%, with readings
including delayed readings at 48 and 96 hours.
286
One patient
had a positive result on the metronidazole SPT and did not
undergo oral challenge. Oral challenge with doses of 250 to 500
mg provoked symptoms within 45 minutes to 1 hour in 2 pa-
tients and after 7 hours in the third. These patients were treated
with intravenous corticosteroids and antihistamines with reso-
lution. Ten control subjects who tested negative on skin prick
testing passed an oral challenge to metronidazole in that
study.
286
These results call into question the utility of skin
testing to metronidazole.
Management. In patients who require metronidazole, but for
whom there is documented positive skin testing result or a high
clinical suspicion for immediate hypersensitivity reaction, and
there are no alternative agents available or the alternative agents
are not preferred (such as for trichomoniasis, as described before),
desensitization can be performed. There are published
TABLE XXX. Protocol for intravenous metronidazole
desensitization*
289
Step Dose Metronidazole concentration (mg/mL) Volume (mL)
15
m
g 0.005 1
215
m
g 0.005 3
350
m
g 0.05 1
4 150
m
g 0.05 3
5 500
m
g 0.5 1
6 1.5 mg 0.5 3
7 5 mg 5.0 1
8 15 mg 5.0 3
9 30 mg 5.0 6
10 60 mg 5.0 12
11 125 mg 5.0 25
12 250 mg 250 mg orally Tablet
13 500 mg 500 mg orally Tablets
14 2000 mg 2000 mg orally Tablets
*Intravenous increments administered at 15- to 20-min intervals. Oral doses
given 1 h apart.
TABLE XXIX. Desensitization protocol for EMB*
271
Solution
(mg/mL) Time (min) Dose (mL) Dose (mg)
Cumulative
dose (mg)
0.01 0 1 0.01 0.01
30 2 0.02 0.03
60 4 0.04 0.07
90 8 0.08 0.15
0.1 120 1 0.1 0.25
150 2 0.2 0.45
180 4 0.4 0.85
210 8 0.8 1.65
1 240 1 1 2.65
270 10 10 12.65
10 300 10 100 112.65
330 30 300 412.65
*Serial dilutions of the 50 mg/5 mL suspension were prepared using purified water.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S37

desensitization protocols for metronidazole via oral desensitiza-
tion and intravenous desensitization.
287-289
Tables XXX and
XXXI list the protocols for intravenous and oral desensitization,
respectively. Desensitization protocols have proven to be very
effective in a case series that found that oral and intravenous
metronidazole desensitization regimens were 100% effective (15
of 15) in the management of trichomonas infection in women
with nitroimidazole hypersensitivity.
290
Patch-test data suggest some cross-reactivity between metro-
nidazole and other imidazoles such as clotrimazole, ketoconazole,
miconazole, and albendazole.
291-293
Therefore, in patients with a
proven hypersensitivity reaction to metronidazole, avoiding
theses agents is recommended if possible.
Antimalarials (by Sara Barmettler, MD)
General.
Antimalarial drugs are used for the treatment and
prophylaxis of malarial infection. Most antimalarial drugs target
the erythrocytic stage of malaria infection, which is the phase of
infection that causes symptomatic illness. There are several
classes of antimalarial drugs including quinoline derivatives,
antifolates, antimicrobials, and artemisinin derivatives. The an-
timicrobials used in malaria treatment and prophylaxis will not
be described in this section.
Major symptoms. Quinoline derivatives include chloro-
quine, quinine, mefloquine, and primaquine. Chloroquine was
one of the first antimalarials produced on a large scale; however,
there is increasing resistance to chloroquine in many malaria-
endemic countries. Commonly described side effects of chloro-
quine include headaches, dizziness, abdominal discomfort,
vomiting, and diarrhea.
294
Chloroquine-induced pruritus has
been described, most frequently in African populations, is tran-
sient (lasting 48-72 hours), and is not responsive to antihista-
mines.
295
Chloroquine has also been described to cause TEN,
and other reactions including fixed drug eruption, bullous
pemphigoid, exfoliative dermatitis, and exacerbation of
psoriasis.
296,297
Oral quinine is associated with cinchonism, which includes a
number of unpleasant adverse effects including nausea, headache,
tinnitus, dysphoria, and blurred vision.
297
Pruritus, skin flush-
ing, and urticaria are associated with quinine hypersensitivity.
Other cutaneous manifestations such as photosensitivity, cuta-
neous vasculitis, and lichenoid photosensitivity after quinine
have been described in case reports.
297
Mefloquine has been associated with a number of adverse
effects including skin reactions such as pruritus (frequency of
4%-10%) and maculopapular rash (frequency of 30%).
298
As-
sociations have also been drawn between mefloquine and urti-
caria, facial lesions, cutaneous vasculitis, SJS, and TEN.
298
Mefloquine is known to cause serious neuropsychiatric toxicity,
including seizures, encephalopathy, and psychosis, which occur
in 0.1% to 5% of patients treated for malaria.
299
Other adverse
effects include vomiting and dizziness.
297
Primaquine is the only 8-aminoquinoline in clinical use and is
generally well tolerated. It can cause hemolytic anemia in patients
with glucose-6-phosphate dehydrogenase deficiency, leukocytosis
and leukopenia, and gastrointestinal upset.
297
Antifolates include sulfonamides (including dapsone), pyri-
methamine, and proguanil. Atovaquone/proguanil is an anti-
malarial combination with good efficacy and tolerability when
used for prophylaxis and treatment. The most common side
effects included headache, gastrointestinal symptoms of abdom-
inal pain, anorexia, nausea, vomiting, diarrhea, and cough.
300,301
Atovaquone has been associated with SJS as well as acute
eosinophilic pneumonia and maculopapular rash.
302,303
One
case of anaphylaxis was described during clinical trials.
304
The
combination of chloroquine and proguanil has also been
described to cause AGEP and cutaneous vasculitis.
305,306
A rare
but serious adverse effect is drug-induced hypersensitivity syn-
drome or DRESS. Up to 3.6% of dapsone recipients develop this
syndrome, and as many as 13% of these die as a result.
307
The artemisinin derivatives have excellent efficacy and safety,
with very few attributable adverse effects.
308
One study reported
an incidence of type I hypersensitivity reactions including urti-
caria and anaphylaxis at 0.03%.
309
Diagnosis. There are rare case reports in which skin testing
was used to evaluate for a type I hypersensitivity reaction to
antimalarial agents. Unfortunately, there is no standardized
nonirritating concentration of these drugs that both uses negative
controls to establish nonirritating concentrations and identifies a
positive case-control with a suggestive history. Specifically, skin
prick testing has been described for atovaquone-proguanil, but
the concentration used was not published and there was no
mention of negative controls tested.
310
Similarly, in another case
report, result of epicutaneous testing performed with chloro-
quine, proguanil, and me fl oquine was negative, but again, there
was no mention of the concentration used or whether there were
negative controls. Patch testing for several antimalarial drugs
(including malarone, proguanil, atovaquone, quinine, chloro-
quine, and mefloquine) has been described, with the drugs
diluted at 30% in a petrolatum base.
303
Management. In most literature describing hypersensitivity
reactions, management includes cessation of the suspected culprit
drug and selection of an alternate therapy. Given the large
number of different classes of medications available for the
treatment and prophylaxis of malaria, the selection of an alter-
native agent with equal efficacy generally has not provided an
unsurmountable challenge, and thus there is a lack of specific oral
challenge protocols or desensitization protocols available for
antimalarial agents.
Antiparasitics (by Sara Barmettler, MD)
General.
Antiparasitics are a class of medications used to treat
diseases caused by nematodes, cestodes, trematodes, amoeba, and
protozoa. The main type of antiparasitic drug is the anti-
helminthic group, which includes the antinematodes and
TABLE XXXI. Protocol for oral metronidazole desensitization*
288
Step Dose (mg)
Metronidazole
concentration (mg/mL) Volume (mL)
Cumulative
dose (mg)
1 0.0025 0.025 0.1 0.0025
2 0.025 0.025 1 0.0275
3 0.25 0.25 1 0.2775
4 2.5 2.5 1 2.7775
5 25 2.5 10 27.78
6 250 250 mg Tablet 277.8
7 750 750 mg Tablets 1027.8
8 1000 1000 mg Tablets 2027.8
*Doses given over the course of 1 d.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S38 BROYLES ET AL
anticestodes. Within the antiparasitics, a number of adverse ef-
fects are associated with each drug, but case reports of hyper-
sensitivity reactions are infrequent.
Major symptoms. Ivermectin is a semisynthetic derivative of
avermectin and is useful in the treatment of a number of parasitic
infections, including onchocerciasis, strongyloidiasis, ascariasis,
trichuriasis, enterobiasis, scabies, head lice, and filarial
worms.
311,312
Adverse effects include gastrointestinal upset,
abdominal pain, fatigue, urticarial or maculopapular rashes,
pruritus, and rarely, hepatotoxicity or neurologic side effects, and
it has been implicated in a mucosal drug eruption with hemor-
rhagic scabs/erosions.
311,313-315
Ivermectin has also been
described to cause a Mazzotti-type reaction (pruritus and aden-
opathy due to dying microfilaria) after the treatment of
onchocerciasis.
311
The benzimidazole class of antiparasitics includes albendazole,
mebendazole, thiabendazole, and triclabendazole. Side effects of
albendazole include abdominal pain, nausea, vomiting, and
diarrhea, as well as rare side effects of transient transaminitis,
agranulocytosis, and urticarial or other dermatologic manifesta-
tions.
316,317
Contact urticaria and contact dermatitis have been
described in association with albendazole.
318
Albendazole has
also been reported to cause fixed drug reaction.
319
The side-effect
profile of mebendazole is similar to that of albendazole and in-
cludes gastrointestinal symptoms such as mild abdominal pain
and diarrhea, as well as urticaria, pruritus, and edema.
320
More
serious cutaneous manifestations have been reported with the
combination of mebendazole and metronidazole, which was
associated with an outbreak of SJS/TEN.
321
A number of adverse
reactions have been described with the use of thiabendazole,
including dizziness, nausea, vomiting, drowsiness, pruritus,
headache, neuropsychiatric disturbances, hepatitis, and hyper-
sensitivity reactions such as SJS.
320,322
Praziquantel has activity against cestodes and trematodes
including parasites such as schistosomiasis, intestinal tapeworms,
cysticercosis, and other flukes. Side effects include headache,
dizziness, drowsiness, nausea, and abdominal discomfort, and less
commonly, itching/rash.
323
Although rare, hypersensitivity re-
actions to praziquantel have been described.
324-327
Interestingly,
mouse model data have suggested that anaphylactic reactions
may be induced by parasite antigen release rather than true hy-
persensitivity reactions to the praziquantel.
328
Diethylcarbamazine is a piperazine derivative with activity
against lymphatic filariasis, loiasis, and visceral larva migrans.
Side effects include fever, headache, dizziness, gastrointestinal
symptoms of abdominal pain and nausea, and urticaria/pruri-
tus.
320
Administration of diethylcarbamazine in the setting of
onchocerciasis is associated with a risk of precipitating the
Mazzotti reaction (which includes symptoms of fever, urticaria,
tender lymphadenopathy, tachycardia, arthralgias, edema,
abdominal pain, and hypotension, and correlates with infection
intensity).
329
Antiprotozoals include eflornithine, melarsoprol, tinidazole,
and metronidazole. Eflornithine is effective in the early and late
central nervous system stage of infections with Trypanosoma
brucei gambiense but not Trypanosoma brucei rhodesiense.
Frequent side effects include diarrhea, anemia, leukopenia, and
hair loss.
330
Melarsoprol is used to treat late-stage African
trypanosomiasis. Use of melarsoprol is limited by its toxicity,
which occurs commonly and includes severe adverse effects such
as encephalopathy, polyneuropathy, exfoliative dermatitis,
myocarditis, and hypersensitivity reactions such as bullous re-
actions.
330,331
Please see separate section on metronidazole for a
detailed description of hypersensitivity reactions associated with
the use of this drug. Antimalarials are also discussed in detail in a
separate section.
Diagnosis. A few case reports describe the use of skin testing
in the evaluation for a type I hypersensitivity reaction to anti-
parasitic agents. Skin prick testing and intradermal testing have
been described for mebendazole and albendazole.
332
However,
despite negative skin prick testing and intradermal testing results
for mebendazole and albendazole, the patient in that case
developed hives on oral challenge with both medications, thus
suggesting a poor predictive value of this skin test.
332
Skin prick
testing and intradermal testing have been described for prazi-
quantel, and results for both were positive in the patient tested;
however, that report did not mention the concentrations used or
whether negative controls were used to establish nonirritating
concentrations of the drug.
325
Patch testing for albendazole has
been described, with 4 of 9 patients tested having positive results,
though notably the patient with a clinical history of contact
dermatitis did not test positive.
318
Management. Management of hypersensitivity reactions in
most cases reported involved cessation of the suspected culprit
drug and selection of alternate therapy. Rarely, case reports have
described desensitization to the antiparasitic agent. Desensitiza-
tion has been described for praziquantel.
324,325
In 1 case, oral
desensitization to praziquantel was attempted using a modified
desensitization protocol with increasing doses of 30, 60, 100,
150, 300, 600, and 1200 mg administered 90 minutes apart.
The patient described in this case did develop symptoms
requiring hydrocortisone, antihistamine, and an H
2
-blocker
treatment during the desensitization, but was able to tolerate
therapeutic doses of praziquantel given for 3 days subse-
quently.
325
In another case, desensitization was attempted using
13 doses of praziquantel at 15-minute intervals (first 6 doses were
18 mg each, the next 3 were 180 mg each, and the final 3 were
360 mg each). In this case, the patient developed generalized
urticaria, difficulty swallowing, and chest tightness within 1 hour
of his last dose. He was able to receive praziquantel on subse-
quent days without symptoms but was also given concomitant
corticosteroids, so it was unclear whether true desensitization was
achieved.
324
Oral challenge has been reported in the case of fixed
drug eruption secondary to albendazole, with cross-reactivity
reported with metronidazole.
319
Antifungals (by Stephanie Logsdon, MD)
General.
Antifungal medications including the azoles have
been used in treatment and prophylaxis for various fungal in-
fections for decades, while newer antifungal medications,
including the echinocandin class, have gained increasingly
widespread use in the treatment of pediatric and adult fungal
infections. The azole drug class is composed of triazoles
(including fluconazole, itraconazole, voriconazole, posaconazole,
and isavuconazole) and imidazoles (including clotrimazole, ke-
toconazole, and miconazole). These drugs disrupt the fungal cell
membrane through impairment of ergosterol synthesis.
333
Each
drug in the azole class has distinctive indications for use, ranging
from mucosal candidiasis to invasive mycoses. These drugs are
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S39
critical for managing the increasing frequency of fungal infections
in all age groups, while offering a cost-effective option for pro-
phylaxis in immunosuppressed patients.
334
Azoles are available in
oral, intravenous, and topical formulations. Ketoconazole and
miconazole are associated with increased rates of toxicity with
administration, while newer triazoles have improved safety pro-
files and reduced drug-drug interactions, and are generally well
tolerated in adult and pediatric patients. All azoles exhibit inhi-
bition of the CYP450 enzymes.
333
The echinocandin drug class includes micafungin and caspo-
fungin, both of which inhibit
b
-(1,3)-D-glucan synthase. The
frequency of invasive fungal infections is increasing in all age
groups, thus escalating the need for effective therapies such as the
echinocandins.
335
These medications have fewer adverse events
and drug-drug interactions, and improved safety profiles over
other antifungal agents. This is important because many patients
who receive micafungin or caspofungin have other serious un-
derlying medical illnesses. Both medications are administered
intravenously, because their large molecular weight precludes
acceptable gastrointestinal absorption. Importantly, neither agent
appreciably affects the SMZ-TMP system, thus reducing the
amount of drug-drug interactions.
Major symptoms of hypersensitivity. Hypersensitivity
reactions have been reported for both azoles and echinocandins.
The most common ADRs to the azole drug class include hepa-
totoxicity, skin exanthems, and gastrointestinal symptoms. In
addition, each individual azole carries a distinctive side-effect
profile.
333,334
Multiple case reports describe TEN, fixed drug
eruptions, and other hypersensitivity reactions including
anaphylaxis to fluconazole, voriconazole, and itraconazole.
336-339
Hypersensitivity to imidazoles, posaconazole, and isavuconazole
is not well described. Common ADRs to the echinocandin drug
class include transaminitis, electrolyte imbalances, fever, and skin
exanthems.
340,341
There are single case reports describing the
development of secondary TEN and hypersensitivity to caspo-
fungin use in adult patients.
342,343
Beyond case reports, hyper-
sensitivity to micafungin and caspofungin is not well described in
the literature.
Diagnosis. Although skin prick testing for azoles is not vali-
dated, case reports have described protocols. Skin testing was
performed in an adult patient with a history of anaphylaxis to
voriconazole and 2 control patients. In this case report, testing
comprised prick testing with 0.5 mg/mL and 0.05 mg/mL vor-
iconazole with negative saline control. The patient had a positive
result with the 0.5-mg/mL dilution, whereas both control pa-
tients had negative results to this dilution.
339
Skin testing to
fluconazole has also been described in case reports. An adult
patient who developed cutaneous and respiratory symptoms
during treatment with fluconazole underwent intradermal skin
testing with 0.2 mg/mL of the drug. Her skin test result was
positive, whereas the skin test result was negative in a nonallergic
control patient.
344
No further skin testing protocols are obtain-
able. Therefore, skin testing may be helpful for the diagnosis of
hypersensitivity to azoles, but further investigation into ideal
concentrations is needed.
Skin prick testing for the echinocandins is not well validated as
well. Only 1 published description of skin testing to caspofungin
was found during a literature review. Skin testing was completed
on an adult patient with history of anaphylaxis to micafungin.
This test included 1 intradermal injection of 0.05 mg caspo-
fungin with a normal saline negative control.
343
No further skin
testing protocols are available. Therefore, obtaining a complete
clinical history remains critical in the diagnosis of echinocandin
hypersensitivity because appropriate skin testing concentrations
remain unclear.
Management. Drug desensitization protocols have been
published for oral fluconazole, oral itraconazole, and intravenous
voriconazole.
336,339
There is extensive variability in the protocols
used for fluconazole desensitization, with protocol durations
ranging from hours to days (Table XXXII).
337,338,344
For IgE-
mediated allergy, preference should be given to desensitizations
that occur over hours, rather than a prolonged protocol. No
intravenous desensitization protocols for fluconazole are pub-
lished. Each protocol was completed without significant systemic
reactions, indicating that desensitization to azoles may be safely
completed. Continued inquiry into the management of hyper-
sensitivity to azole antifungals is necessary, because these medi-
cations continue to be critically required.
Micafungin and caspofungin are becoming critical therapeutic
agents for patients with disseminated or virulent fungal in-
fections, and thus these medications will likely be required in the
future for use in patients who have hypersensitivity reactions to
these medications. Drug desensitization may be an important
tool for these patients. A recently published desensitization
regimen used a standard 12-step protocol that has been suc-
cessfully used with antibiotics such as penicillin
(Table XXXIII).
345
The entire protocol was completed without
reaction, and the patient completed the remainder of her ther-
apeutic course uneventfully. This indicates that rapid de-
sensitizations can be successfully completed in a safe and effective
manner. Strict adherence to the dosing schedule is required to
safely maintain the desensitized state. Further investigation into
these medications will help physicians evaluate and manage
echinocandin hypersensitivities.
Antivirals (by Tito Rodriguez, MD, and Elizabeth
Phillips, MD)
Antiretrovirals
General.
More than 30 antiretroviral (ART) drugs are
currently available in the United States for the treatment of HIV-
1, and these include 6 FDA-approved single-tablet regimens of 3
or more drugs as of March 2016. Six different categories of ART
drugs (nucleoside reverse transcriptase inhibitors, nonnucleoside/
tide reverse transcriptase inhibitors, protease inhibitors [PIs],
entry inhibitors [including fusion inhibitors and CCR5 in-
hibitors], and integrase inhibitors) can be used in combinations
of 3 or more drugs. Current treatment recommendations are to
initiate combination ART drugs in all patients with HIV.
346-348
Nearly all ART drugs have been implicated in hypersensitivity
reactions, usually presenting as delayed onset and ranging from
the usual mild exanthem to less common life-threatening SJS/
TEN or DRESS (Table XXXIV) reactions, with type I hyper-
sensitivity reactions rarely seen.
349
These reactions can be
confused by the presence of underlying opportunistic infections,
immune restoration disease, or the fact that HIV-infected pa-
tients may take other drugs for prophylaxis of opportunistic in-
fections that can also cause the full spectrum of clinically
indistinguishable hypersensitivity reactions (eg, sulfamethoxa-
zole/trimethoprim).
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S40 BROYLES ET AL

Based on current evidence-based me dicine, there should be
no restrictions on who gets ART drugs and this should be
considered in all patients after individual discussion and con-
siderations pre senting with HIV-1 regardless of CD4
þ
T-cell
count, viral load, or AIDS-defini ng i llness history. From ol der
data, hypersensitivity reactions have been described as occurring
100timesmorecommonlyinHIV-infectedpatientsthaninthe
general population, but data from contemporary HIV pop-
ulations are lacking.
349
In general, diagnosis of hypersensitivity
reactions related to HIV therapy is made on the basis of clin ical
presentation, temporal relationship with drug exposure (typi-
cally 1-6 weeks), and e xclusio n of other etiologies.
346
Patients
presenting with mild rash alone without fever or organ
involvement can develop tolerance with continued dosing and
uninterrupted treatment is recomm ended (e g, efavirenz and
PIs).
349,350
Major symptoms and management. Abacavir, a nucle-
oside reverse transcriptase inhibitor, is a common cause of severe
hypersensitivity reactions, occurring in 2.3% to 9% of patients
exposed to the drug.
349
The clinical syndrome of abacavir hy-
persensitivity includes fever, constitutional symptoms, and
gastrointestinal disturbance. Rash is later in onset, and may be
absent in up to 30% of patients.
349
These reactions have been
associated with the presence of the MHC class I allele HLA-
B*5701.
349,351-353
In clinical trials, HLA testing has shown
100% sensitivity of HLA-B*5701 for skin patch-testeconfirmed
abacavir hypersensitivity reactions as well as 100% negative
predictive value; genetic screening for HLA-B*5701 has been
part of guideline-based therapy before abacavir prescription since
2008.
349,351-353
A history of abacavir hypersensitivity reactions is
an absolute contraindication to rechallenge, because subsequent
reactions are often more rapid and severe than the initial reac-
tion.
349,351-353
Particularly with the advent of HLA-B*5701 screening,
contemporary single-tablet regimens used as first-line ART
therapy in clinical practice in the United States are associated
with a very low risk of hypersensitivity reactions.
Abacavir hypersensitivity is unique compared with other drug
hypersensitivity syndromes in that fever and malaise can develop
within the first few days of initial dosing and with relatively late
and inconsistent (70%) appearance of rash. The clinical symp-
toms, signs, and pharmacogenomics of other drug hypersensi-
tivity syndromes are outlined in Table XXXIV.
349
Currently,
abacavir is the only ART drug with an associated hypersensitivity
for which a guideline-approved pharmacogenomic pretreatment
and preventive screening strategy exists.
349
HLA-B*5701 testing
before prescription of abacavir has become consistent practice in
the developed world but is still not widely used in regions where
either abacavir is not widely used (eg, Africa) or where the allele
frequency of HLA-B*5701 is less than 1% (South and Southeast
Asia, excluding Northern Thailand) and India where HLA-
B*5701 carriage rate is as high as 10%.
353
Immediate reactions have been rarely reported, and there are
no standardized protocols for skin prick and intradermal
testing.
354
Antiehepatitis C drugs. Hepatitis C virus (HCV) affects
more than 170 million people globally. Traditional HCV
treatment, which i ncluded pegylated IFN and ribavi rin, had a
low efficacy (45%) and has now been largely replaced by the
direct-acting agents. In all HCV treatment regimens, the rates
of rash were high, noted in up to 20% to 30% of cases.
349
Early direct-acting agents, such as the HCV NS3.4A serine PI
telaprevir developed for genotype 1 HCV infection, in com-
bination with ribavirin and IFN, have now largely been
replaced with newer direct-acting agents. Telaprevir-associated
rash was described in 50% or more of those initiating therapy,
with more than 90% of these being mild to moderate
eczematous rashes that were controlled symptomatically with
antihistamines or topical corticosteroids and resulted in
TABLE XXXII. Fluconazole oral desensitization protocol*
344
Fluconazole 200 mg PO q24h
Full dose 197.76 mg
Step Concentration
Volume
administered (mL)
Dose
administered (mg)
Cumulative
dose (mg)
1 0.02 1.00 0.02 0.02
2 0.02 2.00 0.04 0.06
3 0.02 4.00 0.08 0.14
4 0.2 0.80 0.16 0.30
5 0.2 1.60 0.32 0.62
6 0.2 3.20 0.64 1.26
7 2 0.75 1.50 2.76
8 2 1.50 3.00 5.76
9 2 3.00 6.00 11.76
10 20 0.60 12.00 23.76
11 20 1.20 24.00 47.76
12 20 2.50 50.00 97.76
13 20 5.00 100.00 197.76
PO, Per os (by mouth); q24h, every 24 h.
*Fifteen-minute intervals are given between doses. The first full dose is administered
24 h after completion of step 13.
TABLE XXXIII. Micafungin intravenous desensitization
protocol*
345
Micafungin 150 mg IV q24h
Total to be injected
in each bottle (mg)
Full dose 150 mg
Solution 1 250 mL of 0.006 mg/mL 1.5
Solution 2 250 mL of 0.06 mg/mL 15
Solution 3 250 mL of 0.595 mg/mL 148.82
Step Solution
Rate
(mL/h)
Time
(min)
Administered
dose (mg)
Cumulative
dose (mg)
1 1 2 15 0.003 0.003
2 1 5 15 0.0075 0.0105
3 1 10 15 0.015 0.0255
4 1 20 15 0.03 0.0555
5 2 5 15 0.075 0.1305
6 2 10 15 0.15 0.2805
7 2 20 15 0.3 0.5805
8 2 40 15 0.6 1.1805
9 3 10 15 1.4882 2.6687
10 3 20 15 2.9764 5.6451
11 3 40 15 5.9528 11.5979
12 3 75 186 138.4021 150
Total time 351 min
*The first full dose is administered 24 h after initiation of step 12.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S41

discontinuation in less than 10%. One study showed that in
patients presenting with more than 50% body surface
without DRESS/SJS/TEN, continuation with close mo ni-
toring was a safe alternative.
355
Less common reactions
includedDRESSin5%andSJS/TENinlessthan1%.
Newer direct-acting agents have infrequently been associated
with skin rash. Simeprevir is a second-generation NS3/4A
HCV PI that has been primarily associated with photosen-
sitivity in up to 5% of patients,whichmaybedose-related.
Simeprevir is codosed with sofosbuvir, which was not
associated with phototoxicity in earlier trials when dosed
without simeprevir. There have been no reports of photo-
patch testing that would accurately and reliably differentiate
between phototoxicity and photosensitivity. Reports in the
literature have been mixed, wit h early reports suggesting an
allergic and a lichenoid pattern on histology favoring
phototoxicity.
356,357
Diagnosis of anti-HCV drug hyperse n-
sitivity is based on clinical assessment of the syndrome.
TABLE XXXIV. Clinical manifestations, incidence, and pharmacogenomics of antiviral hypersensitivity syndromes
349
Class Agent Reaction Incidence Treatment limiting HLA association (population)
ART treatments
349
PIs Atazanavir Rash 6% <1% None known
Darunavir Rash 10% <1% None known
SJS/TEN/DRESS <1% 100% None known
Fosamprenavir Rash—moderate to
severe
19% <1% None known
Lopinavir/
ritonavir
Rash 2% <1% None known
Tipranavir Rash 10% <1% None known
NNRTIs Efavirenz Rash 4.6%-20% <2% HLA-DRB1*01 (French)
SJS/TEN/DRESS 0.1% 100% Not known
Etravirine Rash 10% <2% Not known
DRESS/SJS/TEN <0.1% 100% Not known
Nevirapine Rash 4%-38% 6% HLA-B*35:05 (Thai)
HLA-C*04 (African, Asian,
European, Thai)
HLA-DRB1*01 (French)
B*35:05, rs1576*G CCHR1 (Thai)
DRESS Up to 5%;
0.3%-1%
100% HLA-B*14/C*08 (Sardinian/Japanese)
HLA-B*35/C*04 (SE Asian)
HLA-C*04 þ CYP2B6 516 G-/ T
(European/Asian/African)
CYP2B6 (rs2054675, rs3786547,
rs3745274)
HLA-B*35:05/01 (Thai/European)
SJS/TEN 100% CYP2B6 G-/T
HLA-C*04:01 (African/Malawian)
CYP2B6 983 T/C(Mozambique)
Hepatitis <5% HLA-DRB1*01:01 (European)
HLA-DRB1*02:01 (South African)
HLA-B*58:01 (South African)
Rilpivirine Rash 2% <1% None known
Fusion
inhibitors
Enfuvirtide Hypersensitivity
reaction
<1% Mostly for
subcutaneous
reactions not
HSR
None known
NRTIs Tenofovir Rash 5%-7% <1%
None known
Abacavir HSR (fever, GI
symptoms, rash
in 70%)
5%-8% 100%, hypotension,
severe morbidity
on rechallenge
HLA-B*57:01 (European/black)
(100% negative predictive value,
55% positive predictive value)
Rash only 3% <1% Not known
Emtricitabine Pruritus, rash 17%-30% <1% Not known
Integrase
inhibitors
Raltegravir Pruritus, diaphoresis,
rash
2%-7% <1% Not known
DRESS/SJS/TEN <1% 100% HLA-B*53:01 (African ancestry)
933
CCR5
inhibitors
Maraviroc Pruritus 3.8% <1% Not known
GI, Gastrointestinal; HSR, hypersensitivity reaction; NRTI, nucleoside reverse transcriptase inhibitor.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S42 BROYLES ET AL

Other antiviral drugs. Commercially available antivirals for
herpes virus—acyclovir, valacyclovir, famciclovir, and penciclo-
vir—have close chemical structures, and the alternative antiviral
drugs available—foscarnet and cidofovir—present with relevant
side effects. Hypersensitivity reactions to herpes virus antivirals
are infrequent, usually consisting of contact allergy or delayed
systemic reactions most frequently described for acyclovir.
Immediate reactions have been described, but skin testing is
not well standardized and it has been described only in case re-
ports with none or low number of controls.
358
Patch testing has
been shown to be useful in case reports at 10% or higher con-
centration, but there are no data on its sensitivity and predictive
values.
359
Other than abacavir there are no data on the diagnostic
utility of patch testing for other antivirals. Cross-reactivity among
structurally related acyclovir, valaciclovir, and famciclovir is
partial, with approximately 50% of patients tolerating famci-
clovir after reaction to valacyclovir or acyclovir; therefore, chal-
lenge with a suitable alternative after a negative patch-test result
must be considered before desensitization.
360
Hypersensitivity reactions to influenza antivirals are limited to
individual case reports that do not include details on cross-re-
activities or skin testing standardization. Anaphylaxis and delayed
reactions have been reported to oseltamivir.
361
Desensitization. Desensitization protocols to ART drugs
have been described for patients with severe delayed reactions
limited to the skin where the specific antiviral agent was the only
possible treatment for that patient (Table XXXV).
362,363
Although failures have been reported in several case reports with
rapid desensitization protocols to enfuvirtide, it remains the
preferred method due to the unknown risk of resistance with
slow desensitization protocols.
362
If the rapid protocol fails, a
published 2- to 3-day slow desensitization protocol can be
used.
364
Because the mechanism underlying delayed antiviral
hypersensitivity to most antivirals remains unclear, such slow
protocols should be used with caution and only when no alter-
native therapeutic option is possible. Desensitization is contra-
indicated in patients who have experienced severe, life-
threatening immunocytotoxic reactions, vasculitis, or bullous
skin diseases such as SJS/TEN and DHS/DRESS and for aba-
cavir in HLA-B*5701epositive patients.
270
The role of skin testing or desensitization for HCV antivirals
has not been determined, but 2 case reports have described slow
desensitization protocols for ribavirin.
365
Desensitization has
been described in immediate reactions confirmed by challenge
and in some unclear delayed reactions for herpes simplex virus
antivirals. No desensitization protocol to influenza antiviral
treatment has been described.
CHEMOTHERAPEUTIC AGENTS
Carboplatin (by Sarita Patil, MD)
General.
In oncology patients, repeated administration of
platinum-based chemotherapeutic agents can result in the
development of hypersensitivity.
366,367
In particular, the inci-
dence of hypersensitivity reactions increases from 1% after the
first dose of carboplatin to 27% after 7 doses.
366
The peak rate of
hypersensitivity reactions occurs with the eighth or ninth dose,
which often corresponds with the second or third cycle after
restarting treatment for malignancy recurrence.
368
Drug
desensitization has been found to effectively deliver carboplatin
to patients with hypersensitivity.
272
Major symptoms of hypersensitivity. Hypersensitivity
reactions to carboplatin can range from mild cutaneous symp-
toms (flushing, pruritus, urticaria) to systemic anaphylaxis,
defined as involving more than 2 organ systems, often with
cutaneous, gastrointestinal, respiratory, and cardiac symptoms.
Up to 50% of reactions include moderately severe reactions.
272
These reported hypersensitivity reactions do not include SJS,
TEN, erythema multiforme, or serum sickness.
Diagnosis. In addition to a clinical history of reactions
consistent with hypersensitivity, skin testing can aid in the
diagnosis of carboplatin hypersensitivity. Standard carboplatin
skin testing protocols use step-wise skin prick testing (10 mg/
mL) and 3-step intradermal testing (0.1, 1, and either 3 or 5 mg/
mL) with 0.02 mL, in addition to a positive control (0.1 mg/mL
histamine base) and a negative control (saline). Higher intra-
dermal skin testing concentrations (10 mg/mL) cause irritation
and carry a risk of skin necrosis.
368
A positive skin test result can
be defined as a wheal-and-flare reaction, with the wheal’s greatest
diameter being at least 3 mm larger than that seen with saline.
369
Because of potential for false-negative skin testing results in
certain patients, particularly in those with a recent history of
anaphylaxis (within 4-6 weeks of skin testing), repeat skin testing
is recommended for patients whose clinical history is consistent
with a hypersensitivity reaction and who have a negative initial
skin test result on evaluation. These patients’ condition can be
managed with desensitization while they are awaiting repeat skin
testing.
369,370
Management. In patients with carboplatin hypersensitivity,
desensitization with a 12-step protocol (Table XXXVI)ina
monitored setting (in an inpatient setting supervised by an al-
lergy specialist) has been effective in delivering adequate
chemotherapeutic doses to patients requiring therapy. A 13-step
protocol similar to the 12-step protocol, with an additional 60
mL/h step between steps 11 and 12, can also be used.
TABLE XXXV. Rapid subcutaneous (A) enfuvirtide desensitiza-
tion protocol and (B) oral darunavir desensitization protocol
A* B†
Dose Enfuvirtide Dose Darunavir
1 6.25
m
g125
m
g
2 62.5
m
g 2 250
m
g
3 0.125 mg 3 500
m
g
4 0.25 mg 4 1 mg
5 0.5 mg 5 2 mg
6 1 mg 6 5 mg
7 2 mg 7 10 mg
8 5.65 mg 8 25 mg
9 11.25 mg 9 50 mg
10 22.5 mg 10 100 mg
11 45 mg 11 200 mg
12 90 mg 12 300 mg
1-h interval between doses 30-min interval between doses
*Adapted from DeSimone et al.
362
†Adapted from Marcos Bravo et al.
363
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S43
Patients undergoing evaluation with repeat skin testing,
because of a history of hypersensitivity reactions but with a
negative initial skin test result, have also been able to safely
receive their chemotherapeutic dose using a modified 8-step
desensitization protocol (Table XXXVII). Antihistamine pre-
medication is recommended before the use of these desensitiza-
tion protocols.
368,369
Low-risk patients who have a history of nonpruritic, non-
blistering delayed rash or those who have a negative skin test
result between 6 weeks and 6 months after their initial hyper-
sensitivity reactions and who are therefore at a lower risk of
having a falsely negative skin test result may be candidates to
receive carboplatin by infusion at 50% of the standard infusion
rate in an outpatient infusion center setting.
370
For premedication, patients are advised to use 20 mg of oral
cetirizine on the evening before desensitization, the morning of
desensitization, and immediately before initiation of desensi-
tization, but no data are available on optimal premedication
regimens. In addition, before the start of the desensitization
protocol, patients with initial skin symptoms such as pruritus
or urticaria can be given o ral or intravenous diphenhydramine
25 mg and oral ranitidine 150 mg (intravenous ra nitidin e 50
mg or famotidine can be substituted). Patients with mild
cutane ous symptoms (flushing, erythe ma) as part of their hy-
persensitivity reactions may also benefitfrompremedication
with oral aspirin 325 mg on the night before t hei r desensiti-
zation and again 1 hour before their desensitization protocol
begins.
During desensitization, hypersensitivity reactions are treated
with cessation of the protocol and administration of intravenous
diphenhydramine 50 mg for mild reactions. More severe or
recurrent reactions are treated with intravenous methylprednis-
olone 60 mg, and systemic anaphylaxis should be treated with
intramuscular epinephrine 0.3 mg. Once hypersensitivity reac-
tion symptoms resolve, the desensitization can be resumed at a
previous step and completed.
Oxaliplatin (by David Hong, MD)
General.
Oxaliplatin is a third-generation platinum-based
chemotherapeutic agent most commonly used to treat metastatic
colon cancer in combination with leucovorin and fluorouracil
(FOLFOX regimen). As with cisplatin and carboplatin, the risk
of developing type I hypersensitivity reactions increases with
successive exposures. Most patients develop reactions after the
sixth treatment cycle, with the incidence being as high as 20%,
comparable to that of carboplatin (incidence 27% after the sixth
dose).
272
Major symptoms of hypersensitivity. Symptoms of
oxaliplatin hypersensitivity are typical of a mast cellemediated
process; common symptoms include flushing, pruritus, urti-
caria, and back pain. Cardiovascular and respiratory anaphylaxis
is less common but has greater life-threatening potential. Less
commonly, reactions featuring fever and chills/rigors with or
without hypotension, nausea, and diarrhea have been described
in case reports. IL-6 and TNF-
a
levels are often elevated acutely,
and these reactions have been described as “idiosyncratic” or
“cytokine stormelike.”
Diagnosis. Oxaliplatin hypersensitivity appears to be pre-
dominantly IgE-mediated because skin testing using skin prick
and intradermal testing appears to have a high negative predictive
value, ranging from 80% to 100%, and a positive predictive
value of 66% to 80% when skin prick and intradermal testing is
performed. Typically, testing involves skin prick testing with
oxaliplatin at 1 to 5 mg/mL, to be followed, if the result is
negative, by intradermal testing using 10-fold serial dilutions
starting at 10
2
and 10
1
dilutions and undiluted.
371-373
Symptoms of skin irritation, including injection-site pain and
erythema, are more common with higher strengths of intrader-
mal testing. In cases with a clinical history suggestive of type I
hypersensitivity to oxaliplatin but negative skin test result, it may
be appropriate to repeat testing after subsequent infusions of
oxaliplatin, especially if there have been 8 or more previous
lifetime exposures to oxaliplatin.
374
Conversion to a positive skin
test result in such patients would deem them candidates for
desensitization if continuing oxaliplatin therapy is desired.
Management. Type I hypersensitivity to oxaliplatin can be
managed by desensitization in most cases. Various protocols have
been used, with the total duration of infusion ranging from 90
minutes to 16 hours. Castells et al have described the greatest
number of patients desensitized to platin-based chemothera-
peutics using a 12-step protocol with three 10-fold dilutions of
the drug given over approximately 6 hours. An example 12-step
desensitization protocol is included in Table XXXVIII.
Most protocols premedicate patients with antihistamines to
minimize the risk and/or severity of breakthrough reactions with
desensitization. Additional premedication with a combination of
aspirin and montelukast appears to be helpful for those patients
whose reactions have prominently included flushing, thought to
be mediated by the release of prostaglandins, and other inflam-
matory lipid mediators generated as a consequence of mast cell
activation.
The literature describing idiosyncratic/cytokine stormelike
reaction is quite limited, but management seems to revolve
around intense premedication regimens of steroids with or
without antihistamines.
375
Coadministration of intravenous
fluids and infusion rate reduction have also been found to be
useful interventions. The role for desensitization in this clinical
scenario is not yet defined.
Taxanes (by Matthieu Picard, MD)
General.
Immediate hypersensitivity reactions to paclitaxel and
docetaxel occur in around 10% of patients, most commonly on
first exposure, despite premedication with antihistamines and
corticosteroids.
376-378
Nab-paclitaxel and cabazitaxel are less
frequently associated with such reactions.
377
Cremophor-EL
(contained in the paclitaxel formulation) and polysorbate 80
(contained in the docetaxel and cabazitaxel formulation) are
thought to be responsible for immediate hypersensitivity re-
actions because they can cause complement activation and
generate anaphylatoxins.
377
An IgE-mediated mechanism could
also be responsible for some of these reactions.
378,379
Major symptoms of hypersensitivity. Most immediate
reactions are moderate in severity, with the most frequently re-
ported symptoms being skin flushing, dyspnea, chest pain, and
back pain.
376,378
In patients with hypotension, a serum tryptase
level can be measured; an elevated level supports a mast
cellemediated reaction.
378
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S44 BROYLES ET AL

Paclitaxel and docetaxel can cause nonimmediate skin re-
actions with onset from 12 hours to 15 days after the infusion.
378
These reactions are most frequently characterized by flushing or
by a maculopapular skin eruption.
378
Although much less common, severe hypersensitivity reactions
(SJS, TEN, acute interstitial pneumonitis, and subacute cuta-
neous lupus erythematosus) have been described in case reports
in association with paclitaxel, docetaxel, and nab-paclitaxel.
377
Diagnosis. Skin testing can be used to guide the method of
reexposure: desensitization versus challenge in patients with an
immediate taxane-induced hypersensitivity reaction.
378
Con-
centrations used for paclitaxel skin testing range from 1 to 6 mg/
mL (SPT) and from 0.001 to 6 mg/mL (IDT).
378,380
False-
positive results can occur at 6 mg/mL (IDT).
380
Concentrations
used for docetaxel skin testing range from 4 to 10 mg/mL (SPT)
and from 0.04 to 10 mg/mL (IDT).
378,380
Skin testing with
cabazitaxel and nab-paclitaxel has not been reported.
Management. Following an immediate taxane-induced hy-
persensitivity reaction, reexposure through a regular or slowed
infusion with or without additional premedication appears to be
tolerated by many patients.
376,380,381
However, severe reactions
were reported with this approach and desensitization can be used
as an alternative, especially in patients with a positive skin test
response and/or a moderate to severe initial hypersensitivity re-
action (Figure 4).
376,378,380,381
The risk of recurrent reaction
appears to decrease with repeated exposures, and desensitization
protocols can be progressively shortened in patients with good
tolerance to eventually perform a challenge and, if tolerated,
resume regular infusions (Table XXXIX).
378,381
Patients with a delayed skin reaction and a positive skin test
response may be at risk of an immediate hypersensitivity reaction
on reexposure and may require desensitization.
378
The risk of a
recurrent delayed reaction also decreases with repeated exposures
and many can eventually tolerate regular infusions.
378
In
contrast, patients with severe nonimmediate hypersensitivity re-
actions (eg, SJS) should not be reexposed to taxanes.
Methotrexate (by Andrew MacGinnitie, MD, PhD,
and Min Jung Lee, MD)
General.
Methotrexate (MTX) is an antifolate chemotherapy
and immunosuppressant agent that is used for the management
of osteosarcoma, acute lymphocytic leukemia, and rheumatoid
arthritis, along with other autoimmune diseases in adults and
children. It inhibits the activity of dihydrofolate reductase, which
is needed for de novo purine synthesis. Adverse effects, more
commonly seen with high doses used in cancer therapy, include
abdominal cramping, malaise, mucositis, myelosuppression, and
renal and hepatic toxicity.
382
MTX pneumonitis that can prog-
ress to interstitial fibrosis has also been reported.
383
Although the
incidence of hypersensitivity reaction is unknown, it is likely rare,
and case reports of anaphylactic or anaphylactoid reactions have
been described.
384,385
Major symptoms of hypersensitivity. The signs and
symptoms of hypersensitivity reactions have been seen with
standard-dose, high-dose, oral, intramuscular, intrathecal, and/or
TABLE XXXVI. Twelve-step carboplatin desensitization protocol*
Step Solution Concentration (mg/mL) Rate (mL/h) Time (min) Administered dose (mg) Cumulative dose (mg)
1 1 0.02456 2.5 15 0.0154 0.0154
2 1 0.02456 5 15 0.0307 0.0461
3 1 0.02456 10 15 0.0614 0.1075
4 1 0.02456 20 15 0.1228 0.2303
5 2 0.2456 5 15 0.307 0.5373
6 2 0.2456 10 15 0.614 1.1513
7 2 0.2456 20 15 1.228 2.3793
8 2 0.2456 40 15 2.456 4.8353
9 3 2.45666 10 15 6.0916 10.9269
10 3 2.45666 20 15 12.1833 23.1102
11 3 2.45666 40 15 24.3666 47.4768
12 3 2.45666 80 174.375 566.5232 614
*For a total dose of 614 mg carboplatin.
TABLE XXXVII. Eight-step carboplatin desensitization protocol*
Step Solution Concentration (mg/mL) Rate (mL/h) Time (min) Administered dose (mg) Cumulative dose (mg)
1 1 0.1656 5 15 0.207 0.207
2 1 0.1656 10 15 0.414 0.621
3 1 0.1656 20 15 0.828 1.449
4 1 0.1656 40 15 1.656 3.105
5 2 1.64358 10 15 4.109 7.214
6 2 1.64358 20 15 8.2179 15.4319
7 2 1.64358 40 15 16.4358 31.8677
8 2 1.64358 80 174.375 382.1324 414
*For a total dose of 414 mg carboplatin.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S45

intravenous forms of MTX. The reactions can include pruritus,
urticaria, angioedema, wheezing, dyspnea, hypotension, and/or
loss of consciousness.
386,387
Although most reactions occur
subsequently after the first exposure, hypersensitivity reactions
occurring on first exposure have also been described.
388
Diagnosis. The sensitivity and specificity of MTX skin
testing as well as nonir ritating skin testing concentrations have
not been established. However, the following concentrations
have been used for prick testing (10 mg/mL) and intradermal
testing (0.1 mg/mL and 1 mg/mL).
386
Other skin testing
concentrations with IDT (2.5 mg/mL), which w ere nonirri-
tating in 2 healthy controls, have also been used in an adult
patient.
389
Additional case reports in adults have demonstrated
positive prick skin testing result.
390,391
In the pediatric pop-
ulation, a recent retrospective review of a single-center expe-
rience with MTX showed that only 1 of 4 patients tested
positive on skin testing.
386
This may suggest immediate type I
hypersensitivity as part of the pathogenesis in some patients.
Management. For patients with MTX reactions, the man-
agement should be individualized in the context of overall clin-
ical picture after considering risks and benefits of reintroduction.
If the patient has a negative skin testing result and/or history of
minor reaction, challenge may be considered. The challenge can
be performed starting with 1/100th of the total dose. Patients
with severe reactions (especially involving vital sign changes,
pharyngeal edema, respiratory and cardiovascular involvement,
etc) should receive desensitization regardless of the skin test
result. Case reports have described prolonged infusion ranging
from 6 to 27 hours.
392-394
However, at some institutions, the
current practice for both adults and children involves a desen-
sitization protocol with 3 bags of different concentrations that are
administered via 12 steps, with the first bag consisting of 1/100th
of the total dose, the second bag 1/10th of the total dose, and the
third bag containing the remainder of the dose. A 16-step pro-
tocol involving 4 bags has also been performed in cases with
severe reactions. Each step involves doubling of the concentra-
tions every 15 minutes until the last step (step 12) is reached.
The premedications can include H
1
and H
2
antagonists in
addition to leukotriene receptor antagonists and/or corticoste-
roids (Table XL).
Cyclophosphamide (by Rebecca Breslow, MD)
General.
Adverse reactions to cyclophosphamide (CYC) are
most commonly due to drug toxicity. The major determinant is
the cumulative dose received; toxicities are related to both dose
and duration of exposure to this agent. Major toxicities associated
with CYC are bone marrow suppression, increased susceptibility
to infection, gonadal toxicity leading to infertility, increased risk
of hematologic and skin malignancies, cystitis, and bladder
cancer.
395
Less common adverse reactions are described in several
case reports, and are dermatologic. One patient with systemic
lupus erythematosus treated with CYC developed a neutrophilic
eccrine hidradenitis.
396
Another with multiple myeloma (MM)
developed a neutrophilic folliculitis.
397
Type I hypersensitivity reactions to CYC are relatively
rare, but case reports can be found in the literature. Vis-
itsunthorn et al
398
described a pediatric patient treated with
CYC for systemic lupus erythematosus who developed urti-
caria on the fifth exposure to the agent. Skin testing result
was negative, and the pati ent then underwent successful
graded challenge. Rosas et al
399
described another pediatric
patient with acute lymphoblastic leukemia, being treated with
CYC/2-mercaptoethane sulfonate sodium who developed a
maculopapular rash, diaphoresis, respiratory distress, and
TABLE XXXVIII. Twelve-step desensitization protocol for oxaliplatin*
Target dose (mg)
Standard volume
per bag (mL)
Final rate of
infusion (mL/h)
Calculated final
concentration (mg/mL)
Standard time of
infusion (min)
500.0 250 80 2 187.5
Total mg per bag
Solution 1 250 mL of 0.020 mg/mL 5.000
Solution 2 250 mL of 0.200 mg/mL 50.000
Solution 3 250 mL of 1.984 mg/mL 496.065
Step Solution Rate (mL/h) Time (min) Volume infused per step (mL) Dose administered with this step (mg) Cumulative dose (mg)
1 1 2.0 15 0.50 0.0100 0.0100
2 1 5.0 15 1.25 0.0250 0.0350
3 1 10.0 15 2.50 0.0500 0.0850
4 1 20.0 15 5.00 0.1000 0.1850
5 2 5.0 15 1.25 0.2500 0.4350
6 2 10.0 15 2.50 0.5000 0.9350
7 2 20.0 15 5.00 1.0000 1.9350
8 2 40.0 15 10.00 2.0000 3.9350
9 3 10.0 15 2.50 4.9607 8.8957
10 3 20.0 15 5.00 9.9213 18.8170
11 3 40.0 15 10.00 19.8426 38.6596
12 3 80.0 174.375 232.50 461.3405 500.0000
Total time (min) ¼ 339.375 ¼ 5.66 h
*The total volume and dose dispensed are more than the final dose given to patient because many of the solutions are not completely infused.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S46 BROYLES ET AL

anxiety during his fourth cycle of CYC/2-mercaptoethane
sulfonate sodium.
399
The symptoms resolved with steroid
and anti histamine treatment. Skin testing result was also
negative, but on rechallenge, the patient again presented with
a similar rash. He underwent rapid desensitization, which he
tolerated wit hout a reaction, and was able to receive a sub-
seq uent t rea tment 2 days after his desensitization via standard
administration.
Diagnosis. Type I hypersensitivity reaction to CYC is
diagnosed on the basis of clinical history with or without
skin testing. Signs and symptoms consistent with IgE-medi-
ated hypersensitivity, such as hives, flushing, angioedema,
pru ritus, wheezing, an d h ypotension occurring during or
shortly after receiving a dose of CYC, are highly suggestive of
a type I hypersensitivity reaction. Skin testing can be used to
confirm the clinical history; providers perform percutaneous
prick testing with 10 mg/mL and intradermal testing with 1
mg/mL (1:10) and 10 mg/mL (1:1). In the case report by
Visitsunthorn et al,
398
prick and intradermal testing was
performed with 0.02 mL of the following dilutions of CYC:
1:100, 1:10, and 1:1. Rosas et al
399
also performed prick
testing with 10 mg/mL and intradermal testing with 1 mg/
mL. In all cases, prick and intradermal tests with CYC were
compared with a histamine-positive c ontrol and a diluent-
negative control, and results were determined on the basis of
this comparison. Neither of these published studies included
controls to determine o ptimal nonirritating skin testing
concentrations for CYC.
Management. Patients with an equivocal history and negative
skin testing result may be subjected to graded challenge. Vis-
itsunthorn et al
398
described a successful graded challenge to
CYC, in which the patient received antihistamine pretreatment
and was challenged, with doubling of doses every 15 minutes;
however, they did not include the number of doses received and
quantity given with each dose.
Patients with a convincing clinical history with or without a
positive skin test result may undergo rapid desensitization. Pa-
tients may be successfully desensitized using a standard 3-bag 12-
step protocol, with premedication with H
1
and H
2
blockers
(Table XLI).
272
Briefly, bag 1 contains a solution diluted 1:100,
bag 2 a solution diluted 1:10, and bag 3 an undiluted solution.
The rate of infusion is then doubled every 15 minutes until a
threshold dose is reached; the patients may then receive the
remainder of the undiluted bag 3 at a final rate of infusion of 80
mL/h. The protocol reported by Rosas et al
399
used an initial
dose of 0.12 mg CYC diluted 1:4000, doubled every 15 to 30
FIGURE 4. Approach to taxane reintroduction in patients with hypersensitivity reaction. Mild immediate hypersensitivity reactions
present with symptoms that are limited to the skin (eg, flushing) or that involve a single organ/system and that are mild (eg, mild back
pain). Moderate hypersensitivity reactions present with symptoms that involve at least 2 organs/systems (eg, flushing and dyspnea) but
without a significant drop in blood pressure or in oxygen saturation. Severe hypersensitivity reactions present with symptoms that
typically involve at least 2 organs/systems and with a significant drop in blood pressure (systolic 90 mm Hg and/or syncope) and/or in
oxygen saturation (92%). In patients with a hypersensitivity reaction with desensitization or challenge, premedication can be adjusted
for the next procedure, which can be administered using either the same or a longer protocol. Patients in whom the hypersensitivity
reaction does not recur can then be treated with a shorter desensitization protocol, challenge, or regular infusion according to the al-
gorithm. To ensure the patient’s tolerance, each procedure can be repeated several times before proceeding with a shorter desensitization
protocol, challenge, or regular infusion.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S47
minutes until a 1500-mg dose was delivered, for a cumulative
dose of 2500 mg.
Doxorubicin (by Kathleen Lee-Sarwar, MD)
General.
Doxorubicin is an inhibitor of DNA and RNA syn-
thesis that intercalates between DNA base pairs, inhibits topo-
isomerase II, and leads to production of free radicals.
Doxorubicin is available without liposomal encapsulation (trade
name Adriamycin), in liposomal form (trade name Myocet), and
in pegylated liposomal form (trade name Doxil). Liposomal
encapsulation allows for preferential concentration in tumor
tissue.
The nonliposomal form of doxorubicin is very rarely associ-
ated with hypersensitivity reactions. In contrast, the incidence of
hypersensitivity reactions to pegylated liposomal doxorubicin is
approximately 8%, with some series reporting up to a 25%
incidence.
400
Reactions usually occur during the first cycle. The
mechanism of hypersensitivity on first exposure may be
explained at least in part by complement activation, which has
been demonstrated in vivo during infusion of pegylated lipo-
somal doxorubicin and may lead to mast cell activation.
400
Interestingly, in the setting of carboplatin hypersensitivity,
combining carboplatin with pegylated liposomal doxorubicin
appears to have a protective effect and is associated with reduced
incidence of hypersensitivity reactions compared with carbopla-
tin as a single agent or in combination with paclitaxel.
401
Major symptoms of hypersensitivity. Typical clinical
features include those of mast cellemediated reactions such as
flushing, pruritus, urticaria, dyspnea, sense of doom, and hypo-
tension. Macular eruption and chest pain or back pain may also
occur.
272
Hypersensitivity reaction severity ranges from mild to
life-threatening.
Doxorubicin may also cause adverse effects that mimic, but
are not, hypersensitivity reactions. Liposomal doxorubicin is
associated with palmar-plantar erythrodysesthesia, also called
hand-foot syndrome, which is a relatively common dermatologic
toxic reaction associated with cytotoxic chemotherapy that can
limit the use of such drugs. Definitive prevention and treatment
strategies for palmar-plantar erythrodysesthesia have not yet been
established. This typically occurs in the first 3 cycles of treat-
ment.
402
Other cutaneous adverse effects include diffuse follic-
ular rash, intertrigo-like eruption, and radiation recall
dermatitis.
403
Acute cardiotoxicity is rare and may present with
arrhythmias such as atrial fibrillation, acute heart failure,
myocarditis, or acute myocardial infarction. Chronic cardiomy-
opathy is a more common and dose-limiting adverse effect.
Diagnosis. The ability to perform skin testing in the evalua-
tion of doxorubicin hypersensitivity is limited by cutaneous
toxicity.
404
Doxorubicin hypersensitivity is therefore generally
diagnosed clinically, and there are no published skin testing
protocols at this time.
Management. Desensitization has been successfully per-
formed in patients with hypersensitivity reactions to doxorubicin
and is an option for management when there is no effective
alternative treatment.
271
In a report of 413 cases of rapid
desensitization, desensitization to doxorubicin was successfully
completed in a total of 29 patients. A 12-step rapid desensiti-
zation protocol may be used in cases with mild or moderate
symptoms (Table XLII).
272
In the case of severe reactions
including hypoxemia or hypotension, a 16-step rapid desensiti-
zation protocol should be used for the patient’s first lifetime
desensitization. If this is tolerated, a 12-step rapid desensitization
protocol may be considered for subsequent infusions. Standard
pretreatment includes a histamine H
2
-receptor antagonist and a
long-acting histamine H
1
-receptor antagonist, with optional
additional premedications tailored to the patient’s initial
reaction.
Pemetrexed (by Paige Wickner, MD)
General.
Pemetrexed is an antifolate agent approved for use in
patients with lung cancer and malignant pleural mesothelioma.
The exact incidence of hypersensitivity reactions to pemetrexed is
unknown. In phase III trials, 17% to 22% of patients receiving
this agent developed a rash.
405
The literature includes case re-
ports of pemetrexed-induced pneumonitis, pemetrexed-induced
urticarial vasculitis, angioedema, AGEP, and anaphylactic
reactions.
Diagnosis. The clinical symptoms should be closely evaluated
and if IgE is suspected, skin testing and desensitization can be
considered. If a more serious reaction occurred that is not felt to
be IgE-mediated, alternative treatment options should be
considered. Intradermal skin testing results with pemetrexed at
dilutions of 1:10,000, 1:1,000, 1:100, and 1:10 have been re-
ported. However, more data are needed to determine optimal
nonirritating skin test concentrations.
Management. For patients with suspected IgE-mediated re-
actions with no reasonable alternatives, desensitization has been
successfully reported (Table XLIII).
Lenalidomide (by Paige Wickner, MD)
General.
Lenalidomide is an antiangiogenic agent that is pri-
marily used for the treatment of MM, myelodysplastic syndrome,
and mantle cell lymphoma. The exact incidence of hypersensi-
tivity reactions to lenalidomide is unknown. The literature re-
ports rashes occurring in 29% to 43% of patients, depending on
underlying disease indication. A spectrum of rashes has been
reported, including facial swelling, SJS, erythema multiforme,
neutrophlic predominant rashes, and urticaria. Timing of re-
actions has been reported as both acute and delayed in onset.
Diagnosis. Clinical symptoms should be reviewed, evaluated,
and continued, or alternative treatment options discussed. At
present, there are no published nonirritating skin test concen-
trations for lenalidomide.
Management. There are case reports of desensitization to
lenalidomide. An outpatient desensitization protocol used a
starting dose of 2.5 mg and desensitized 5 patients to lenalido-
mide without adverse events. A shortened version of the outpa-
tient desensitization can be repeated if the first desensitization
has no adverse events (Tables XLIV and XLV). There are pub-
lished rapid desensitization protocols for typical IgE-mediated
symptoms as well as noneIgE-mediated symptoms.
406
Patients
with evidence or suggestion of SJS/TEN or medication-induced
laboratory abnormalities are not good candidates for
desensitization.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S48 BROYLES ET AL

TABLE XXXIX. Examples of desensitization and challenge protocols for paclitaxel (135-175 mg/m
2
) infused every 3 wk over 3 h
(example ¼ 294 mg)
Four-bag/16-step protocol
Bag Volume per bag (mL) Concentration per bag (mg/mL) Amount of bag infused (mL) Dose infus ed per bag (mg)
Solution 1 250 0.00118 9.38 0.011
Solution 2 250 0.0118 9.38 0.111
Solution 3 250 0.118 18.75 2.213
Solution 4 250 1.167 250 291.665
Step Solution Rate (mL/h) Time (min) Volume infused (mL) Dose infused per step (mg) Cumulative dose (mg)
1 1 2.5 15 0.625 0.001 0.001
2 1 5 15 1.25 0.001 0.002
3 1 10 15 2.5 0.003 0.005
4 1 20 15 5 0.006 0.011
5 2 2.5 15 0.625 0.007 0.018
6 2 5 15 1.25 0.015 0.033
7 2 10 15 2.5 0.030 0.063
8 2 20 15 5 0.059 0.122
9 3 5 15 1.25 0.148 0.270
10 3 10 15 2.5 0.295 0.565
11 3 20 15 5 0.590 1.155
12 3 40 15 10 1.18 2.335
13 4 10 15 2.5 2.917 5.252
14 4 20 15 5 5.834 11.086
15 4 40 15 10 11.667 22.753
16 4 80 174.4 232.5 271.262 294.0
Total time (h) ¼ 6.67
Three-bag/12-step protocol
Bag Volume (mL) per bag Concentration (mg/mL) per bag Amount of bag infused (mL) Dose infused per bag (mg)
Solution 1 250 0.0118 9.38 0.111
Solution 2 250 0.118 18.75 2.213
Solution 3 250 1.167 250 291.676
Step Solution Rate (mL/h) Time (min) Volume infused (mL) Dose infused per step (mg) Cumulative dose (mg)
1 1 2.5 15 0.625 0.007 0.007
2 1 5 15 1.25 0.015 0.022
3 1 10 15 2.5 0.030 0.052
4 1 20 15 5 0.059 0.111
5 2 5 15 1.25 0.148 0.259
6 2 10 15 2.5 0.295 0.554
7 2 20 15 5 0.590 1.144
8 2 40 15 10 1.18 2.324
9 3 10 15 2.5 2.917 5.241
10 3 20 15 5 5.834 11.075
11 3 40 15 10 11.667 22.742
12 3 80 174.4 232.5 271.328 294.0
Total time (h) ¼ 5.67
Two-bag/8-step protocol
Bag Volume (mL) per bag Concentration (mg/mL) per bag Amount of bag infused (mL) Dose infused per bag (mg)
Solution 1 250 0.118 18.75 2.213
Solution 2 250 1.167 250 291.787
Step Solution Rate (mL/h) Time (min) Volume infused (mL) Dose infused per step (mg) Cumulative dose (mg)
1 1 5 15 1.25 0.148 0.148
2 1 10 15 2.5 0.295 0.443
3 1 20 15 5 0.590 1.033
4 1 40 15 10 1.18 2.213
(continued)
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S49

Thalidomide (by Margee Louisias, MD, MPH)
General.
Thalidomide is an immunomodulatory drug with
antiangiogenic properties.
407
It is approved for use in erythema
nodosum leprosum and MM, with off-label use in many con-
ditions such as HIV apthous ulcers and graft-versus-host dis-
ease.
408
It is administered alone for erythema nodosum or in
conjunction with dexamethasone for MM. Because of its known
severe teratogenic effects it can be prescribed and dispensed only
by prescribers and pharmacies enrolled in the THALOMID
REMS program in the United States.
409
It is available as capsules
in the United States. It is soluble at 25
C in dimethyl sulfoxide
and sparingly soluble in water and ethanol. The incidence of
thalidomide hypersensitivity reactions ranges from 6.3% to
47.7% among several thalidomide clinical trials.
407
Major symptoms. Thalidomide hypersensitivity presents
with delayed (usually within first month of treatment) skin re-
actions of varying severity.
407
However, in a retrospective review,
skin reactions were seen more than 12 weeks after initiation with
thalidomide or thalidomide analogs (lenalidomide, pomalido-
mide), in the setting of dose escalation or steroid tapering.
410
Dry skin, pruritic maculopapular rash, morbilliform rash, diffuse
erythematous eruptions, scaling erythroderma, eosinophilia, and
TEN/SJS have been described in the literature.
407,411
Body
distribution is variable but is reported to mostly affect the trunk
and proximal limbs.
407
Diagnosis. There is no standardized skin testing or patch
testing protocol available. Nucera et al
412
performed prick-by-
prick testing with a solution made from a tablet dissolved in
saline. However, because thalidomide (capsule) is soluble only in
dimethyl sulfoxide, which is a skin irritant, skin prick testing can
be done only with thalidomide dissolved in saline in accordance
with European Network of Drug Allergy/EAACI (ENDA/
EAACI) guidelines.
79
Nucera et al
412
also performed intradermal
testing with thalidomide at a concentration of 0.04 mg/dL. Patch
testing was also done according to ENDA/EAACI and American
Academy of Dermatology guidelines. Patches were applied to the
interscapular area with commercially available adhesive anallergic
gauze strips and assessed at 48 and 72 hours.
269,413
Saline and
histamine were used as negative and positive controls for all
testing, respectively; however, whether these concentrations are
nonirritating was not established.
Management. The challenge protocol given in Table XLVI is
adapted from Nucera et al
412
who performed it in an ambulatory
setting. The patient experienced a pruritic, erythematous rash
after completion of day 2. Symptoms improved with an anti-
histamine. The desensitization protocol is also adapted from
Nucera et al,
412
who performed it over 5 days in a hospital
setting (Table XLVII). The same patient underwent desensiti-
zation without adverse reaction and completed treatment
without any issues. Oral antihistamine was used as
premedication.
Tyrosine kinase inhibitors (by Joyce Hsu, MD)
Since the introduction of imatinib in 2001, the tyrosine kinase
inhibitor (TKI) family of therapeutics has continued to expand
rapidly. Currently, there are 31 FDA-approved medications in
this family, including (with targets) the following
414,415
:
ALK: crizotinib, ceritinib, alectinib, and brigatinib
BCR-Abl: bosutinib, dasatinib, imatinib, nilotinib, and
ponatinib
BTK: ibrutinib
c-Met: crizotinib and cabozantinib
Epidermal growth factor receptor (EGFR) family: gefitinib,
erlotinib, lapatinib, vandetanib, afatinib, and osimertinib
JAK family: ruxolitinib and tofacitinib
PDGFR alpha/beta: axitinib, gefitinib, imatinib, lenvatinib,
nintedanib, pazopanib, regorafenib, sorafenib, and sunitinib
RET: vandetanib
Src family: bosutinib, dasatinib, ponatinib, and vandetanib
Vascular EGFR family: axitinib, lenvatinib, nintedanib,
regorafenib, pazopanib, sorafenib, and sunitinib
TKIs are used in the treatment of numerous malignancies
and myeloproliferative disorders, as well as hypereosinophilic
syndrome and aggressive systemic mastocytosis in the case of
imatinib. As a family, they are associated with significant
cutaneous and systemic side effects that are important to
differentiate from true hypersensitivity.
TABLE XXXIX. (Continued)
Step Solution Rate (mL/h) Time (min) Volume infused (mL) Dose infused per step (mg) Cumulative dose (mg)
5 2 10 15 2.5 2.917 5.130
6 2 20 15 5 5.834 10.964
7 2 40 15 10 11.667 22.631
8 2 80 174.4 232.5 271.369 294.0
Total time (h) ¼ 4.67
Challenge protocol
Bag Volume per bag (mL) Concentration per bag (mg/mL) Amount of bag infused (mL) Dose infus ed per bag (mg)
Solution 1 250 1.176 250 294
Step Solution Rate (mL/h) Time (min) Volume infused (mL) Dose infused (mg) per step Cumulative dose (mg)
1 1 2 15 0.5 0.588 0.588
2 1 8 15 2 2.352 2.940
3 1 80 185.625 247.5 291.06 294.0
Total time ¼ 3.59 h
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S50 BROYLES ET AL

Skin rash—often erythematous and papular or pustular—is
commonly associated with TKIs, particularly EGFR inhibitors
(up to 100% of patients).
416
Because of the association between
EGFR- and vascular endothelial growth factor and its
receptoreassociated rash development and improved survival
outcomes, supportive care with steroids, antihistamines, and
antibiotics is preferable in many cases.
417
Hand-foot skin reac-
tion, characterized by pain and blistering on the palms and soles,
is associated with sorafenib, sunitinib, and other vascular EGFR
inhibitors. It occurs generally in the first 2 to 4 weeks of treat-
ment. Oral dysesthesia and mucositis occurs with TKIs, partic-
ularly sorafenib and sunitinib, and may occur simultaneously
with skin rash and hand-foot syndrome.
418
Mucosal involvement
may also include conjunctivitis with EGFR inhibitors and peri-
orbital edema with imatinib. These symptoms can be difficult to
differentiate from systemic drug reactions, and skin biopsy can be
helpful.
419
Diarrhea is also observed commonly with TKIs as a class.
Several of the TKIs, such as lapatinib, sunitinib, and pazopanib,
carry a black box warning for liver toxicity, but it occurs
commonly with other TKIs including gefitinib.
420
Dose reduc-
tion or drug discontinuation should be considered with hepa-
totoxicity as well as severe and chronic diarrhea.
419
Desensitization protocols have been described for several TKIs.
Imatinib. Ten patients with imatinib hypersensitivity who
underwent subsequent desensitization were described by Nelson
et al
421
in 2006. These patients had predominantly cutaneous
initial reactions, though several had additional fever, blistering,
edema, or diarrhea. One patient was skin tested by skin prick
testing at 0.01 and 0.1 mg/mL and intradermal testing at 0.001
mg/mL, 0.01 mg/mL, and 0.1 mg/mL. Erythema was reported
on intradermal testing. Of these 10 patients who completed a 4-
hour rapid oral desensitization (Table XLVIII), 8 were subse-
quently able to tolerate daily imatinib without hypersensitivity
symptoms. The other 2 patients developed rash hours to days
after desensitization.
Subsequently, Paolo
422
published a case report describing
slow oral desensitization to imatinib over 23 days for a patient
who developed eosinophilic dermatitis after 6 weeks
(Table XLIX). Skin testing result was negative, using skin prick
0.1 mg/mL, and intradermal testing with 0.0001 mg/mL,
0.001mg/mL,0.01mg/mL,and0.1mg/mLimatinib.Patch
testing was also performed, and the result was negative using
0.1 mg/mL imatinib in 5% petrolatum. For the rapid oral
desensitization, sam e-day dose increases were administered
every 20 minutes. The patient initially failed rapid desensiti-
zation twice, but was able to tolerate imatinib after slow oral
desensitizati on (Table XLIX).
Crizotinib. Rash w ith crizotin ib occurs in about 10% of pa-
tients, and is generally mild to moderate in severity. Hyper-
sensitivity reactions are rare, but reported. Sanchez-Lopez
et al
423
described a patient presenting with urticaria and facial
edema f or more than 40 days after initiation of treatment. Skin
testing w as performed with crizotinib capsules suspended in
water (skin prick 25 mg/mL, intradermal testing 0.025 mg/mL
and 0.25 mg/mL) and the result was negative. Five normal
controls also had negative results to skin testing with t his
regimen. The patient was premedicated with intramuscular
dexchlorpheniramine 5 mg and methylprednisolone 40 mg, and
the 5-step oral desensitization was performed with 30-minute
intervals and 120 minutes of observation on completion
(Table L). The patient subsequently tolerated crizotinib
without hypersensitivity symptoms.
In 2014, Awad et al
424
also described 2 patients with more
rapid-onset crizotinib hypersensitivity, one with immediate ur-
ticaria and edema and the other with a delayed maculopapular
rash. Patients were premedicated 1 hour and 12 hours before
desensitization with 10 mg of loratadine and 10 mg of cetirizine.
Both patients were successfully desensitized to crizotinib using a
12-step rapid desensitization protocol, with 15-minute intervals
between steps (Table LI).
Sorafenib. Sorafenib is more commonly associated with hand-
foot skin reaction and other symptoms related to toxicity, but it
can rarely (<1%) trigger allergic symptoms, including urticaria.
In 2008, Bauer et al
425
described a patient who developed a
pruritic generalized maculopapular rash after 2 weeks of sorafenib
therapy. The rash resolved with discontinuation of sorafenib and
treatment with oral antihistamines and topical steroids. They
TABLE XL. Samples of 2 desensitization protocols for MTX*
Step
MTX
(mg/mL)
Volume
infused per
step (mL)
Dose
administered
with this step (mg)
Cumulative
dose (mg)
Twelve-step protocol
1 0.002 0.5 0.001 0.001
2 0.002 1.25 0.0025 0.0035
3 0.002 2.5 0.005 0.0085
4 0.002 5 0.01 0.0185
5 0.02 1.25 0.025 0.0435
6 0.02 2.5 0.05 0.0935
7 0.02 5 0.1 0.1935
8 0.02 10 0.2 0.3935
9 0.2 2.5 0.4961 0.8896
10 0.2 5 0.9921 1.8817
11 0.2 10 1.9843 3.866
12 0.2 232.5 46.134 50
Sixteen-step protocol
1 0.0002 0.5 0.0001 <0.001
2 0.0002 1.25 0.00025 <0.001
3 0.0002 2.5 0.0005 0.001
4 0.0002 5 0.001 0.002
5 0.002 0.5 0.001 0.003
6 0.002 1.25 0.0025 0.005
7 0.002 2.5 0.005 0.010
8 0.002 5 0.01 0.020
9 0.02 1.25 0.025 0.045
10 0.02 2.5 0.05 0.095
11 0.02 5 0.1 0.195
12 0.02 10 0.2 0.395
13 0.2 2.5 0.4961 0.891
14 0.2 5 0.9921 1.883
15 0.2 10 1.9843 3.867
16 0.2 232.5 46.134 50.000
*Goal dose of MTX was 50 mg via intravenous administration. Premedications
include H
1
and H
2
antagonists with or without a leukotriene receptoreblocking agent
with or without cortico steroids. Interval of 15 min between steps and the rate of
infusion at the last step is continued until the end of infusion.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S51

were able to restart sorafenib using premedication and a 6-day
oral tolerance protocol, with day 1 doses given every 15 minutes
(Table LII, A). The patient developed erythema after the 11-step
protocol, which was treated with 4 mg dimethindene maleate,
with resolution. The patient was premedicated with methyl-
prednisolone 24 mg on day 1, and premedicated with methyl-
prednisolone 24 mg and fexofenadine 180 mg on days 2 to 12.
Thereafter, fexofenadine was used only as needed.
More recently, Linauskiene et al
426
described a patient with
sorafenib treatment complicated by fever and generalized urti-
caria 10 days into treatment. The urticaria resolved 5 days after
discontinuation of sorafenib, systemic glucocorticoids, and oral
antihistamines. The 8-day oral tolerance protocol (Table LII, B)
was based on the Bauer et al
425
study, and was complicated by
antihistamine-responsive pruritus during the first several days of
the protocol.
426
This patient also developed facial urticaria after
TABLE XLI. Twelve-step desensitization protocol for CYC*
Target dose (mg)
Standard volume
per bag (mL) Final rate of infusion (mL/h)
Calculated target
concentration (mg/mL)
Standard time of
infusion (min)
1000 250 80 4 187.5
Total mg per bag
Solution 1 250 mL of 0.040 mg/mL 10.000
Solution 2 250 mL of 0.400 mg/mL 100.000
Solution 3 250 mL of 3.969 mg/mL 992.130
Step Solution Rate (mL/h) Time (min) Volume infused per step (mL) Dose administered with this step (mg) Cumulative dose (mg)
1 1 2.0 15 0.50 0.0200 0.0200
2 1 5.0 15 1.25 0.0500 0.0700
3 1 10.0 15 2.50 0.1000 0.1700
4 1 20.0 15 5.00 0.2000 0.3700
5 2 5.0 15 1.25 0.5000 0.8700
6 2 10.0 15 2.50 1.0000 1.8700
7 2 20.0 15 5.00 2.0000 3.8700
8 2 40.0 15 10.00 4.0000 7.8700
9 3 10.0 15 2.50 9.9213 17.7913
10 3 20.0 15 5.00 19.8426 37.6339
11 3 40.0 15 10.00 39.6852 77.3191
12 3 80.0 174.375 232.50 922.6809 1000.0000
Total time (min) ¼ 339.375 ¼ 5.66 h
*The total volume and dose dispensed are more than the final dose given to patient because many of the solutions are not completely infused.
TABLE XLII. Example 12-step/3-bag desensitization doxorubicin protocol
Target dose 58 mg
Standard volume per bag 250 mL
Solution Volume per bag (mL) Concentration (mg/mL) Total dose per bag (mg)
Solution 1 250 0.00232 0.58
Solution 2 250 0.0232 5.8
Solution 3 250 0.23017 57.543
Step Solution Rate (mL/h) Time per step (min) Dose administered per step (mg) Cumulative dose (mg) Fold increase per step
1 1 2.5 15 0.0015 0.0015 NA
2 1 5 15 0.0029 0.0044 2
3 1 10 15 0.0058 0.0102 2
4 1 20 15 0.0116 0.0218 2
5 2 5 15 0.029 0.0508 2.5
6 2 10 15 0.058 0.1088 2
7 2 20 15 0.116 0.2248 2
8 2 40 15 0.232 0.4568 2
9 3 10 15 0.5754 1.0322 2.48
10 3 20 15 1.1509 2.183 2
11 3 40 15 2.3017 4.4848 2
12 3 80 175 53.5152 58 2
Total time ¼ 5.66 h
NA, Not applicable/available.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S52 BROYLES ET AL

reaching the maintenance dose of 400 mg twice daily, which was
managed with dose reduction to 400 mg in the morning and 200
mg in the evening. Premedication with prednisolone 30 mg and
bilastine 20 mg was used on days 1 to 4, and premedication with
bilastine 20 mg on days 5 to 7.
Sunitinib. Generalized hypersensitivity reactions are rare with
sunitinib. Bar-Sela et al
427
described a patient presenting with
onset of generalized acute urticaria and facial edema 4 days after
starting sunitinib. The patient was desensitized 1 week after
resolution of his symptoms, with oral premedications including
prednisone 20 mg and promethazine 25 mg given 1 hour before
initiation. Each of the 10 steps was carried out in 1-hour in-
tervals, and he was observed overnight before discharge
(Table LIII). The patient required desensitization with this
protocol twice, because of a treatment pause. A mild pruritic rash
developed after the first desensitization, but resolved with oral
steroid and antihistamine, and he was able to continue the
medication. The second desensitization was well tolerated, and
he subsequently was continued on sunitinib treatment without
hypersensitivity reactions.
Alectinib. In one report, a patient developed a skin rash
affecting the arms and trunk starting 10 days after initiation of
alectinib.
428
Skin biopsy was notable for perivascular infiltration
of histiocytes, neutrophils, and lymphocytes in the upper dermis.
The rash resolved completely after discontinuation of alectinib,
and the patient underwent successful oral desensitization using a
protocol that was administered over 9 days (Table LIV).
BIOLOGICALS
Rituximab (by Patrick Brennan, MD, and Meredith
Dilley, MD)
General.
Rituximab is a chimeric mouse-human mAb directed
against CD20, an abundant surface receptor found on B lym-
phocytes. The incidence of clinically significant reactions to rit-
uximab is very high, and varies widely with the indication for
treatment. Most studies have reported reaction rates during the
initial infusion ranging from 25% to more than 75%.
429-433
The
incidence of reactions during subsequent infusions is much
lower.
430,432
It should be noted, however, that severe infusion
reactions can also occur only after several exposures, or on
reexposure after a long hiatus. Most infusion reactions respond to
symptomatic treatment and/or lowering of the infusion rate, and
most reactions can be managed by the supervising oncologist or
rheumatologist. Only a fraction of infusion reactions to ritux-
imab warrant evaluation and treatment by an allergy specialist.
Because of the high incidence of reactions, however, identifying
cases that are appropriate for referral can be challenging. An al-
lergist’s evaluation is recommended for patients who have
experienced reactions that are consistent with immediate-type
hypersensitivity, severe reactions, repeated reactions, or progres-
sive reactions with multiple medication administrations.
Major symptoms of hypersensitivity. Common infu-
sion reactions can involve symptoms that are consistent with
immediate-type hypersensitivity, including hypotension, bron-
chospasm, angioedema, urticaria, flushing, rhinitis, and pruritus.
The use of rituximab for lymphoid malignancy also often pre-
cipitates malaise, fever, and chills, possibly secondary to tumor
lysis. In autoimmune diseases, headache, nausea, and diarrhea
have been commonly reported. The allergy specialist should also
be aware of the possibility for serious, nonimmediate adverse
reactions including serum sickness, SJS, TEN, myocardial
infarction, arrhythmia, shock, and pulmonary toxicity.
434
Diagnosis. Reactions to rituximab can be IgE-mediated. In
patients with suspected rituximab hypersensitivity referred to an
academic allergy practice, skin testing result for rituximab was
reported as positive in 6 of 9 patients tested, suggesting that at
least some reactions to rituximab may be IgE-mediated.
435
A case
report has more thoroughly demonstrated the presence of anti-
rituximab IgE in a patient who had experienced progressive
hypersensitivity symptoms after rituximab exposure.
436
The use
of a BAT has also been reported for reactions to rituximab, but
further study is required before this modality can be used in
clinical practice.
437
As a practical approach, skin testing to rit-
uximab using 10 mg/mL epicutaneously, followed by 0.01 mg/
mL, 0.1 mg/mL, and 1 mg/mL intradermally, is recommended.
It should be noted that systematic testing of nonirritating con-
centrations for most mAbs has not been reported. A positive skin
test result after a reaction consistent with immediate hypersen-
sitivity should be interpreted as confirmation of allergy.
TABLE XLIV. Rapid lenalidomide desensitization protocol
Time (min) Dilution (mg/mL) Volume (mL)
0 0.025 mg/mL 0.01
30 0.05
60 0.1
90 0.5
120 1
150 5
180 3.34
210 0.25 mg/mL 1
240 2
270 3
300 4
330 1.50 mg/mL 1
360 2
390 6
TABLE XLIII. Desensitization protocol for pemetrexed
Step Solution* Infusion rate (mL/h) Infusion time (min)
1C 5 15
2 C 10 15
3 C 20 15
4 C 50 15
5 C 100 17.25
6 B 20 15
7 B 50 15
8 B 100 19.5
9 A 20 15
10 A 50 15
11 A 100 15
12 A 200 15.6
*Solution A: pemetrexed 815 mg þ normal saline 100 mL; solution B: pemetrexed
40.75 mg þ normal saline 50 mL; solution C: pemetrexed 4.075 mg þ normal saline
50 mL.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S53

However, a negative skin testing result should not dismiss the
possibility of immediate hypersensitivity, because the sensitivity
of this testing is not known. In addition, there may be non eIgE-
mediated reactions to rituximab infusion that involve mast cell
degranulation and mediators of allergic disease, and these re-
actions would also be amenable to the management approaches
described herein. Therefore, a careful history of the reaction(s)
experienced remains a valuable diagnostic tool.
Management. Although not standardized, both antihista-
mines and systemic glucocorticoids are routinely used as ritux-
imab premedications for both oncologic and autoimmune
indications. If a reaction occurs, the infusion should be stopped,
and symptoms treated. For nonanaphylactic reactions, after
symptoms have resolved, the infusion can generally be restarted
at a slower rate. Because most infusion reactions occur during the
first exposure and are not seen with subsequent exposures,
standard reinfusion after a first-exposure infusion reaction is
reasonable in the absence of a life-threatening reaction.
In a retrospective review of 67 patients who reacted to rit-
uximab, 63% of reactions occurred during the first exposure; of
these, 88% were grade 1 or 2.
438
Of these patients, 88% were
rechallenged with rituximab on the same day, and those with a
grade 1 reaction were able to complete the infusion. However, all
4 patients with a grade 3 reaction had a reaction during rechal-
lenge. After a grade 2 reaction, 84% tolerated same-day chal-
lenge, but 5 patients had a subsequent mild reaction.
For patients referred for evaluation by an allergist, skin testing
can be useful in guiding management.
439,440
If skin testing result
is negative after a mild to moderate reaction that was consistent
with immediate hypersensitivity, a graded dose challenge can be
considered; 1/100 and 1/10 dose challenges before the full dose,
with a 1-hour interval, are recommended. For patients with
positive skin testing result, or for those with negative skin test
results who experienced severe reactions consistent with imme-
diate hypersensitivity, rapid desensitization to rituximab is rec-
ommended. Reinstituting treatment with mAbs is often urgent
and time-sensitive, making the process of evaluation with skin
testing difficult because of time limitations. When treatment
options are limited, desensitization should be considered to allow
for the continued use of these drugs in patients who have a re-
action consistent with immediate hypersensitivity. Rapid desen-
sitization has been a highly successful strategy for preventing
immediate-type hypersensitivity reactions to rituximab and other
mAbs.
440
In 2 case series of 14 and 7 adult patients with clinical
histories consistent with rituximab hypersensitivity, all were
successfully desensitized.
435,441
Successful desensitization has
subsequently been reported with multiple protocols.
442
A 12-
step protocol for desensitization of adults is recommended for the
initial desensitization (Table LV).
Successful rapid desensitization to rituximab and other mAbs
has also been reported in pediatric populations.
443-445
In a pe-
diatric case series, rituximab desensitization was successfully used
in 3 patients and a total of 17 infusions.
444
For an adolescent
TABLE XLVI. Thalidomide challenge protocol
Day Time (h) Dose (mg)
10 1
0.5 2
13
1.5 4
Observation for 6 h
20 25
0.5 25
150
Observation for 6 h
TABLE XLV. Slow desensitization to lenalidomide and schematic illustration of desensitization schedule*
Week† Monday Tuesday Wednesday Thursday Friday Saturday Sunday
1 2.5 mg
2 2.5 mg 2.5 mg 2.5 mg
3 2.5 mg 2.5 mg 2.5 mg 2.5 mg
4 2.5 mg 2.5 mg 2.5 mg 5 mg 2.5 mg 5 mg 2.5 mg
5 5 mg 5 mg 5 mg 5 mg 5 mg 5 mg 5 mg
6 10 mg 5 mg 10 mg 5 mg 10 mg 10 mg/d
Step Lenalidomide dose
1 2.5 mg PO during outpatient clinic visit and observation for 1 h. No further doses 1wk
2 2.5 mg PO every 3 d 3 doses
3 2.5 mg PO every other day 3 doses
4 2.5 mg PO qd 3 doses
5 2.5 mg PO alternating with 5 mg PO 6 doses
6 5 mg PO qd 6 doses
7 5 mg PO alternating with 10 mg PO 6 doses
8 10 mg/d*
PO, Per os (by mouth); qd, every day.
*This is an example using a target dose of 10 mg. Weekly complete blood cell counts with differential and liver function tests were obtained during desensitization.
†This regimen can be altered depending on the final target dose. For example, a target dose of 25 mg could be achieved by alternating the 10-mg dose with the 20-mg dose for a
few doses, then introducing 25 mg/d. Once the patient has tolerated slow desensitization, repeat desensitization can be done over a 1-wk period, starting at 2.5 mg 2d,5mg
2 d, 7.5 mg 2 d, and then 10 mg/d.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S54 BROYLES ET AL

patient in this series, a similar 12-step protocol was used suc-
cessfully (Table LVI). Two younger patients, however, experi-
enced significant reactions when a 12-step protocol was used. For
these patients, the desensitization protocol was successfully
adapted to adjust for the patients’ weight, with rate increases of
not more than approximately 0.5 mg/kg/h, and with the final
infusion rate not exceeding 2 mg/kg/h.
Cetuximab (by Timothy Kyin, MD)
Cetuximab is a chimeric mouse-human IgG
1
mAb against
EGFR approved in 2005 for use in the United States for the
treatment of metastatic colorectal cancer and squamous cell
carcinoma of the head and neck. According to the product in-
formation, 3% of patients will experience a severe allergic reac-
tion, with 90% of these reactions occurring on first exposure.
Symptoms can include difficulty breathing, low blood pressure,
shock, loss of consciousness, and/or heart attack.
Since its release, it has become evident that the rate of first-
exposure hypersensitivity reaction is higher than previously
described, with a regional preference for the southeastern United
States. A 2007 review of patients receiving treatment with
cetuximab at major centers in North Carolina and Tennessee
showed that a grade 3 to 4 hypersensitivity reaction occurred in
19 (22%) of 88 patients.
446
Another multicenter study
demonstrated that most patients who experienced a hypersensi-
tivity reaction had preformed IgE to cetuximab without any
previous exposure. The antibody was speci fi c for galactose-
a
-1,3-
galactose, which is present in the Fab portion of the cetuximab
heavy chain.
447
Unlike most mAbs, cetuximab is generated in a
mouse cell line (SP2/0), which expresses the gene for
a
-1,3-
galactosyltransferase. Investigation of this regional variation in
reaction rates led to the discovery that tick bites are the cause of
IgE production to galactose-
a
-1,3-galactose.
448
This antibody
has also been linked to delayed red meat anaphylaxis.
449
Some
have suggested that evaluation of preexisting anticetuximab IgE
could be used as a screening tool for pretreatment risk
stratification.
450
Current manufacturer guidelines recommend discontinuation
of cetuximab after a severe allergic reaction, but there have been
reports of successful desensitizations to this medication. The first
such report was in 2009 in a 60-year-old woman with metastatic
breast cancer who had hypotension, tachycardia, and oxygen
desaturation after receiving only 28 mg of a planned 843-mg
dose during her first exposure.
451
She was later found to have
preexisting IgE to cetuximab. She was subsequently able to un-
dergo a complete desensitization with a 5-bag, 20-step protocol.
At Brigham and Women’s Hospital, in conjunction with the
Dana Farber Cancer Institute, a modified rapid desensitization
has been used (unpublished data). This was in a 49-year-old
woman with first-exposure hypersensitivity, involving urticaria,
nausea, vomiting, and throat-closing sensation, and who had a
positive intradermal skin test result at 1:10 dilution. A modified
1-bag, 5-step protocol with manipulation of the infusion rate was
used for desensitization in this patient (Table LVII). Because of
the proprietary nature of the cetuximab solution, there was
concern about compound stability if the cetuximab was diluted
with standard buffers. She has thus far completed 8 de-
sensitizations of cetuximab with this protocol without
complications.
Therefore, based on these experiences, it seems reasonable that
desensitization is a viable option for this medication in the
appropriate clinical setting.
Other mAbs (by Matthieu Picard, MD)
TNF-
a
inhibitors
General.
TNF-
a
inhibitors are used for the treatment of in-
flammatory bowel disease, rheumatoid arthritis, ankylosing
spondylitis, and psoriasis.
452-456
This class of biologics includes
infliximab, certolizumab, adalimumab, golimumab, and
etanercept.
Major symptoms of hypersensitivity. Immediate hy-
persensitivity reactions to infliximab: Immediate hypersensitivity
reactions to infliximab occur in about 10% of patients and
present with features that can suggest mast cell/basophil activa-
tion (flushing, pruritus, dyspnea, dizziness/hypotension) as well
as with nonspecific symptoms (headache, increased blood pres-
sure, chest and back pain, fever, and chills).
435,445,457-459
Im-
mediate hypersensitivity reactions may occur with the first
exposure but their incidence peaks around the seventh
infusion.
435,457
Injection-site reactions and systemic reactions to certolizumab,
adalimumab, golimumab, and etanercept: Injection-site reactions
occur in about 20% of patients treated with adalimumab or
etanercept and in 2% to 6% of patients treated with certolizu-
mab or golimumab.
460,461
Reactions to etanercept occur in
median on the fourth injection, have their onset 1 to 2 days after
injection, last 2 to 3 days, and may be accompanied by recall
TABLE XLVII. Thalidomide desensitization protocol
Day Time (h) Dose
1 0 0.1
m
g
0.5 0.2
m
g
1 0.3
m
g
1.5 0.4
m
g
Observation for 1 h
201
m
g
0.5 2
m
g
13
m
g
1.5 4
m
g
Observation for 1 h
3 0 0.1 mg
0.5 0.2 mg
1 0.3 mg
1.5 0.4 mg
Observation for 1 h
401mg
0.5 2 mg
13mg
1.5 4 mg
210mg
2.5 20 mg
330mg
3.5 40 mg
Observation for 1 h
5 0 100 mg
Observation for 1 h
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S55

reactions (local reactions at the site of previous injections).
461
These reactions usually wane over time.
461
Systemic reactions
have rarely been reported with TNF-
a
inhibitors that are
administered subcutaneously.
462
Nonimmediate hypersensitivity reactions: Serum
sicknesselike reactions have been described with infliximab and
adalimumab although they are much less frequent than imme-
diate hypersensitivity reactions.
460
Onset is typically from 5 to 7
days after infusion, and the hypersensitivity reaction may involve
fever, malaise, arthralgia/arthritis, and an erythematous
(sometimes) urticarial skin eruption.
463
Psoriasiform and
eczematous skin eruptions may develop during treatment with
TNF-
a
inhibitors.
464
Other rare reactions that may occur
include symmetrical drug-related intertriginous and flexural ex-
anthem, erythema multiforme, SJS, and various autoimmune
diseases such as drug-induced lupus.
465-468
Diagnosis. IgE-mediated reactions account for a subset of
immediate hypersensitivity reactions to infliximab and may be
the cause of some injection-site reactions and also of rare sys-
temic reactions to adalimumab and etanercept.
445,462
A positive
immediate skin test response to infliximab and anti-infliximab
IgEs were seen on average in 28% (range, 4%-67%) and 21%
(range, 13%-27%) of reactive patients, respectively.
435,445,469-471
Suggested skin testing concentrations for the various TNF-
a
inhibitors are presented in Table LVIII. In patients with a
negative skin test result, an IgG-mediated mechanism may be
responsible for the hypersensitivity reaction. Patients with anti-
infliximab IgGs are at increased risk (relative risk varies from 2.4
to 4.0) of immediate hypersensitivity reactions compared with
patients without such antibodies.
472,473
TABLE XLVIII. Imatinib rapid oral desensitization*
421
Step Concentration Volume (mL) Dose (mg)
1 10 ng/mL 1, 2, 4 0.00007
2 100 ng/mL 1, 2, 4 0.00075
31
m
g/mL 1, 2, 4 0.0075
410
m
g/mL 1, 2, 4 0.075
5 100
m
g/mL 1, 2, 4 0.75
6 1 mg/mL 1, 2, 4 7.5
7 10 mg/mL 1, 2, 4 75
8 100-mg tablet 1 100
9 100-mg tablet 2 2 200
*Each step was administered sequentially in 15-min intervals to the 200-mg dose. An additional 100 mg was given at home the evening after desensitization, and the
maintenance dose of 400 mg daily was started the next morning.
TABLE XLIX. Imatinib slow oral desensitization*
422
Day Concentration Volume (mL) Daily dose (mg)
1 1 mg/mL 1, 2, 4 7
2-4 10 mg/mL 1, 2, 4 70
5-7 100-mg tablet 1 tablet 100
8 100-mg tablet 1 tablet 107
1 mg/mL 1, 2, 4
9-11 100-mg tablet 1 tablet 170
10 mg/mL 1, 2, 4
12-14 100-mg tablet 2 tablets 200
15 100-mg tablet 2 tablets 270
10 mg/mL 1, 2, 4
16-21 100-mg tablet 3 tablets 300
22 100-mg tablet 3 tablets 370
10 mg/mL 1, 2, 4
23 100-mg tablet 4 tablets 400
*Daily doses were administered once daily. When multiple doses were administered on the same day, each step was administered in 20-mi n intervals.
TABLE L. Crizotinib rapid oral desensitization*
423
Step Dose (mg)
110
215
325
450
5 100
*Each step was administered sequentially in 30-min intervals.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S56 BROYLES ET AL

Management. When treatment is indicated, patients with an
immediate hypersensitivity reaction to infliximab should prefer-
ably be reexposed through desensitization (Table LIX) if skin
testing result is positive or the initial hypersensitivity reaction was
severe or they experienced recurrent hypersensitivity reactions
despite added premedication and/or a slowed infusion
rate.
435,459,469,470,474
In others, re-treatment using the protocols
developed by Cheifetz et al can be used and appear to be
safe.
459,469,475
These protocols vary depending on the severity of
the initial reaction and consist of slowly increasing the infusion
rate after administering premedication (H
1
antihistamine, acet-
aminophen, and corticosteroids for severe reactions) (Figure 5).
The risk of a recurrent reaction with subsequent exposures is
around 33% on the first reexposure and progressively decreases
thereafter.
475
Therefore, in patients who tolerate desensitization
well, it may be reasonable to progressively shorten desensitization
protocols and eventually proceed to a challenge procedure except
in those with an IgE-mediated allergy (Figure 6).
476
Anti-infliximab antibodies do not cross-react with adalimu-
mab.
477
However, patients who develop anti-infliximab anti-
bodies are more prone to develop antiadalimumab antibodies
and have a higher treatment failure rate with adalimumab.
461
Also, rare cases of patients with immediate hypersensitivity re-
action to infliximab have been described who also reacted to
adalimumab (injection-site reaction or serum sicknesselike re-
actions).
469,478
Despite these caveats, switching to adalimumab
after a hypersensitivity reaction to infliximab can be
attempted.
459,469
Injections-site reactions to etanercept, adalimumab, golimu-
mab, or certolizumab are usually treated with ice, an oral anti-
histamine, topical corticosteroids, and analgesics.
460
Desensitization could be considered for those with important
injection-site reactions or with systemic reactions (Table LX).
462
Desensitization is not a safe method of preventing non-
immediate hypersensitivity reactions to TNF-
a
inhibitors, but
the optimal approach remains unclear. Switching to another
TNF-
a
inhibitor has not been adequately studied, though some
authors have reported that reexposure to the suspected culprit
drug may not necessarily lead to a recurrent reaction.
465,479
Ofatumumab and obinutuzumab. Ofatumumab and
obinutuzumab target CD20 and are used in the treatment of B-
cell malignancies.
480-482
Immediate hypersensitivity reactions,
thought to be caused by a cytokine release syndrome, occur in
more than 50% of patients treated with 1 of these mAbs on first
exposure.
481,483,484
Although the incidence of hypersensitivity
reactions rapidly decreases with subsequent infusions, severe
reactions that led to drug discontinuation and even fatal hy-
persensitivity reactions, in the case of ofatumumab, have been
reported.
481,483,485
Skin testing to these mAbs has not been
studied. The approach used for hypersensitivity reactions to
rituximab can be applied to these agents because at least 2 pa-
tients were successfully desensitized to obinutuzumab
(Figure 6).
474,476
Brentuximab vedotin. Brentuximab vedotin is a chimeric
mAb coupled with a microtubule-disrupting agent used in the
treatment of CD30
þ
lymphomas.
486
Several cases of anaphylaxis
have been reported, which usually occurred after at least 1 un-
eventful exposure.
487-489
Skin testing to brentuximab vedotin
could help identify patients with an IgE-mediated allergy.
474
Desensitization appears to be the method of choice for reexposure
in patients with anaphylaxis (Table LIX)(Figure 6).
474,487-489
Trastuzumab. Trastuzumab is a humanized mAb against the
human EGFR 2 used in the treatment of breast cancer.
490
Sixteen percent to 40% of patients experience an immediate
hypersensitivity reaction during their first exposure to trastuzu-
mab, with clinical features suggestive of a cytokine release syn-
drome.
491,492
Most of these patients tolerate future infusions
after premedication with an antihistamine and a corticoste-
roid.
490-492
Patients who experience an immediate hypersensi-
tivity reaction after tolerating several infusions of trastuzumab are
likely to have developed an IgE-mediated reaction to the mAb.
Skin testing (Table LVIII) can be useful to identify those pa-
tients.
435,470
Although most patients with a mild to moderate
hypersensitivity reaction due to cytokine release will tolerate
reexposure through a standard reinfusion, desensitization
(Table LIX) should be considered for those with a severe cyto-
kine release reaction or with an IgE-mediated reaction
(Figure 6).
435,470,474,491,492
Papulopustular acneiform skin
eruptions have been reported in some patients treated with
trastuzumab. All were able to continue treatment with trastu-
zumab and responded well to acne treatment.
493
Pertuzumab. Pertuzumab is a humanized mAb that,
compared with trastuzumab, recognizes a different epitope of
human EGFR 2.
494
These 2 mAbs are used in combination in
the treatment of breast cancer.
494-496
Very few patients experi-
ence immediate hypersensitivity reactions to pertuzumab.
495
However, in a recent case report, a patient with anaphylaxis on
second exposure to pertuzumab was successfully reexposed
through desensitization.
497
Skin testing result to pertuzumab was
negative (Table LVIII), but a BAT result was positive, suggesting
an IgE-mediated mechanism.
497
Therefore, desensitization
(Table LIX) could be considered for patients with suspected IgE-
mediated reactions to this mAb even in the absence of a positive
skin test result (Figure 6).
474,476
Bevacizumab. Bevacizumab is a humanized mAb used in the
treatment of many types of cancer that targets vascular endo-
thelial growth factor.
498
Immediate hypersensitivity reactions to
bevacizumab occur in less than 3% of patients and are usually
mild.
499
Of the rare patients with an immediate hypersensitivity
reaction, most tolerate subsequent exposures with premedication
TABLE LI. Crizotinib rapid oral desensitization*
424
Step Concentration (mg/mL) Volume (mL) Dose (mg)
1 0.05 1.25 0.0625
2 0.05 2.5 0.125
3 0.5 0.5 0.25
4 0.5 1 0.5
5 0.5 2 1
6 5 0.4 2
7 5 0.8 4
8 5 1.6 8
9 5 3.2 16
10 5 6.25 31.25
11 5 12.5 62.5
12 5 25 125
*Each step was administered sequentially in 15-min intervals.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S57

with an antihistamine and a reduction in the infusion rate.
500
However, in 2 recent studies, two-thirds of patients with an
immediate hypersensitivity reaction to bevacizumab had a
positive skin test result (Table LVIII) and all underwent suc-
cessful desensitization (Table LIX).
470,474
Therefore, an IgE-
mediated mechanism should be suspected in those with recurrent
hypersensitivity reactions or with a severe immediate hypersen-
sitivity reaction (Figure 6).
474,476
Tocilizumab. Tocilizumab is a humanized mAb that binds
to IL-6 receptors to block IL-6 signaling.
501
It is used to treat
rheumatoid arthritis and systemic or polyarticular juvenile
arthritis.
501
Immedi ate hype rsensitivity reactions to tocilizu-
mab are rare, but several cases of anaphylaxis have been re-
ported occurring from the second exposure onward.
502,503
Skin
testing (Table LVIII) is usually useful in identifyin g patients
with an IgE-mediated hypersensitivity reaction, and desensiti-
zation (Table LIX) should be considered the method of choic e
to reexpo se those patients to tocilizumab
(Figure 6).
470,474,502, 503
Omalizumab. Omalizumab is a humanized mAb that binds
free IgE antibodies.
504
It is administered subcutaneously and is
used in the treatment of moderate to severe allergic asthma and
chronic idiopathic urticaria.
504
Anaphylaxis to omalizumab is
rare and is estimated to occur in 0.1% to 0.2% of patients.
505
A
history of anaphylaxis, regardless of the cause, was recently
shown to increase the risk of developing anaphylaxis to omali-
zumab to 0.6%.
506
More than half the cases of anaphylaxis to
omalizumab occur less than 1 hour after medication adminis-
tration. However, delayed reactions ( >24 hours after the injec-
tion) and a protracted course, with symptoms progressively
developing over the course of several hours, have been re-
ported.
506,507
Two patients with symptom onset more than 24
hours after the injection were rechallenged and both had recur-
rent reactions, strengthening the causality of omalizumab in these
reactions.
507
The mechanism of immediate hypersensitivity re-
actions to omalizumab remains elusive. Skin prick testing pro-
tocols with full-strength concentration followed by intradermal
testing with 1:10,000 dilution have been reported.
508
Serum
tryptase was normal in all patients with anaphylaxis to omalizumab
in whom it was measured.
509,510
Specific IgE or IgG against
omalizumab were not detected in 21 cases of anaphylaxis.
507
Skin
testing result to nonirritating concentrations of omalizumab was
negative in all patients tested (Table LVIII).
506
For patients with
hypersensitivity reactions to omalizumab, the approach remains
uncertain, but rechallenge is not recommended especially in pa-
tients with severe reactions.
476
Desensitization (Tables LXI and
LXII) may be attempted, followed by weekly or biweekly admin-
istration to preserve the desensitized state and may allow for
continued administration in some patients.
474,511
Insulin (by Christina Yee, MD, PhD)
General.
Adverse reactions to insulin have decreased in fre-
quency since the introduction of synthetic recombinant DNA
human insulin analogues, but recombinant humanized insulin
can still be associated with hypersensitivity reactions. Prevalence
of suspected insulin allergy has been reported in as few than 3%
of patients annually.
512
Pediatric and adult patients with either
type 1 or type 2 diabetes may be affected.
Recombinant human insulin analogues such as lispro, aspart,
and glulisine (short-acting) or detemir and glargine (long-acting)
have decreased immunogenicity compared with bovine or porcine
TABLE LII. Sorafenib oral tolerance induction
Day Step Dose (mg) Intervals
A
425
1 1 0.4 Every 15 min
2 0.8
3 1.6
4 3.2
56
612
724
850
9 100
10 200
11 400
2 1 100 mg Every 2 h
2 100 mg
3 200 mg
4 200 mg
3-5 1 200 mg Every 2 h
2 200 mg
3 200 mg
4 200 mg
6 and onwards 1 400 mg Every 12 h
2 400 mg
B
426
1 1 0.4 Every 15 min
2 0.8
3 1.6
4 3.2
56
612
724
850
9 100
10 200
11 400
2 1 100 mg
2 100 mg 3 h later
3 200 mg 2 h later
3 1 100 mg Every 2 h
2 200 mg
3 200 mg
4 1 200 mg Every 2 h
2 200 mg
3 200 mg
5-7 1 200 mg Every 2 h
2 200 mg
3 200 mg
4 200 mg
8 and onwards 1 400 mg Every 12 h
2 400 mg
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S58 BROYLES ET AL

insulin preparations. Short-acting insulin preparations are believed
to be the least frequently associated with sensitivity because the
rapidity of absorption is thought to decrease immune
exposure. Allergic sensitization may also occur because of insulin
additives or materials used to administer insulin injections or
infusions.
Insulin reactions can pose significant challenges for diabetic
patients, who may have no alternatives to lifelong insulin therapy.
Major symptoms of hyperse nsitivity. Adverse reactions
to insulin may present as immediate-type (IgE-mediated) hy-
persensitivity, delayed-type hypersensitivity, serum sickness, or
other reactions.
Immediate-type hypersensitivity reactions (type I, or IgE-
mediated) are the most commonly reported insulin reactions.
The causative antigen may be the insulin itself or other com-
ponents of the preparation. Symptoms may encompass the full
TABLE LIII. Sunitinib rapid oral desensitization*
427
Step Concentration (mg/mL) Volume (mL) Dose (mg)
1 0.2 0.25 0.05
2 0.2 0.5 0.1
3 0.2 1 0.2
4 0.2 2.5 0.5
51 11
61 22
71 44
81 88
9 16-mg capsule 1 capsule 16
10 25-mg capsule 1 capsule 25
*Each step was administered sequentially in 60-min intervals.
TABLE LIV. Alectinib slow oral desensitization*
428
Day Dose (mg)
1, 2 40
3, 4 80
5, 6 160
7, 8 300
9 Twice-daily 300
*Each step was administered daily, with the exception of day 9 and on.
TABLE LV. Rituximab desensitization protocol (1000 mg)*
Solution 1 250 mL of 0.040 mg/mL
Solution 2 250 mL of 0.400 mg/mL
Solution 3 250 mL of 3.969 mg/mL
Step Solution Rate (ml/h) Time (min) Volume infused per step (mL) Dose administered with this step (mg) Cumulative dose (mg)
1 1 2.0 15 0.50 0.02 0.02
2 1 5.0 15 1.25 0.05 0.07
3 1 10.0 15 2.50 0.10 0.17
4 1 20.0 15 5.00 0.20 0.37
5 2 5.0 15 1.25 0.50 0.87
6 2 10.0 15 2.50 1.00 1.87
7 2 20.0 15 5.00 2.00 3.87
8 2 40.0 15 10.00 4.00 7.87
9 3 10.0 15 2.50 9.92 17.79
10 3 20.0 15 5.00 19.84 37.63
11 3 40.0 15 10.00 39.69 77.32
12 3 80.0 175 232.50 922.68 1000.00
*Total time 5 h 40 min.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S59

spectrum of immediate-type reactions, from mild pruritus or rash
to generalized urticaria or to dyspnea, hypotension, or other
symptoms of anaphylaxis. Localized symptoms may be most
prominent near the site of the insulin injection or infusion.
Systemic reactions are rare, but anaphylaxis to insulin has been
reported even with recombinant insulin preparations.
513-515
Immediate-type insulin allergy frequently occurs within several
weeks after initiation of insulin therapy or after restarting ther-
apy, but it can also develop after years of treatment.
Symptoms of insulin reactions may also be delayed in pre-
sentation. In the case of immune complexemediated (type III)
reactions, subcutaneous nodules may develop at the injection site
after 2 to 6 hours, with later symptoms of serum sickness
including pruritic rash, joint pains, fatigue, and elevated in-
flammatory markers.
516
Delayed-type hypersensitivity (type IV)
reactions to insulin detemir have also been reported, with
symptoms of prolonged induration, pain, warmth, and swelling
at the injection site, or, in 1 case report, leukoclastic vascu-
litis.
517,518
Delayed-type hypersensitivity reactions may also
occur in response to components of insulin preparations, such as
protamine or metacresol or from components of equipment used
for insulin administration, such as adhesives or tubing.
519,520
Insulin resistance and lipoatrophy at injection sites were re-
ported in patients receiving bovine or porcine insulin, and were
linked to circulating antibodies to insulin.
521
Transition to re-
combinant human insulins has eliminated many impurities
associated with animal insulin preparations, and such associated
side effects are now rare.
Diagnosis. A careful history is vital in the evaluation for a
potential insulin reaction. Other causes of symptoms must be
excluded. In a retrospective study, 59% of suspected cases of
insulin “allergy” did not have an allergic cause.
522
Patients with
diabetes mellitus have increased incidence of other autoimmune
diseases, which should also be considered on the differential
diagnosis. Chronic urticaria, atopic dermatitis, psoriasis, vascu-
litis, and other systemic autoimmune diseases may present with
rash or other skin manifestations.
Timing and characteristics of associated symptoms should be
considered to determine the likely type of reaction. Allergic re-
actions to insulin can occur to the insulin itself; to preservatives
and solvents such as metacresol, phenol, or sodium phosphate; or
to insulin additives such as protamine or zinc, which help slow
insulin absorption.
523-526
In addition, hypersensitivity reactions
have been reported to injection or infusion pump equipment,
latex, nickel, epoxy resin, and local disinfectants.
527,528
There-
fore, consideration must also be given to components of the
insulin preparation being used, as well as to components of
equipment used for infusion (syringes, needles, infusion pump
tubing, adhesives, etc), latex in this equipment or in the stopper
of insulin vials, and possible reactions to alcohol or disinfectant
wipes.
TABLE LVI. Rituximab desensitization protocol for pediatric patients*
444
Solution Total volume (mL) Drug per bag (mg) Concentration (mg/mL)
1 250 2.06 0.008
2 250 20.6 0.082
3 250 205.189 0.821
Step Solution Rate(mL/h) Rate (mg/kg/h) Time (min) Dose per step (mg) Cumulative dose (mg)
1 1 1 0.0006 15 0.0021 0.0021
2 1 2.5 0.002 15 0.0052 0.0072
3 1 5 0.003 15 0.0103 0.0175
4 1 10 0.006 15 0.0206 0.0381
5 2 2.5 0.02 15 0.0515 0.0896
6 2 5 0.03 15 0.103 0.1926
7 2 10 0.07 15 0.206 0.3986
8 2 20 0.1 15 0.412 0.8106
9 3 5 0.3 15 1.0259 1.8366
10 3 10 0.7 15 2.0519 3.8885
11 3 20 1.3 15 4.1038 7.9922
12 3 30 2 482.5 198.0078 206
*Total infusion time: 648 min; final infusion rate: 2.0 mg/kg/h.
TABLE LVII. Cetuximab desensitization protocol: 1-solution, 5-step protocol*
Step Solution Rate (mL/h) Time (min) Volume infused per step (mL) Dose administered with this step (mg) Cumulative dose (mg)
11† 5.0 30 2.50 5.0658 5.0658
2 10.0 30 5.00 10.1316 15.1974
3 20.0 30 10.00 20.2632 35.4606
4 40.0 30 20.00 40.5264 75.9870
5 80.0 256.87 342.49 694.0011 769.9881
*Target dose: 770.0 mg; volume per bag: 380 mL; final rate of infusion: 80 mL/h; calculated final concentration: 2.026 mg/mL; total time: 376.87 min (6.28 h).
†Solution 1: 380 mL of 2.026 mg/mL (total per bag: 770.002 mg).
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S60 BROYLES ET AL
Although diagnostic testing for anti-insulin IgE and IgG
4
antibodies has been studied, the presence of insulin-specific an-
tibodies cannot be used to establish a diagnosis of insulin allergy,
because anti-insulin IgE antibodies can be present in patients
with no apparent allergy.
529
BATs have also been used to mea-
sure in vitro responses to insulin, but their clinical utility has not
been established, and the tests are not commercially available.
Skin testing for the insulin preparations used, alternative in-
sulin preparations, and additives may help distinguish the specific
allergenic component or components (Table LXIII). Skin testing
for short-acting insulins (Table LXIV), which may be less aller-
genic, may be considered for patients with possible allergic
symptoms to continuous subcutaneous insulin infusion, or pa-
tients with allergy to other forms of insulin for whom this
therapy may be considered.
Positive insulin skin testing results have been reported in
patients receiving insulin without allergic symptoms, so skin test
results must be correlated with clinical symptoms.
522
It may be
helpful to include alternative insulin preparations in skin testing,
to identify preparations to which a patient may not be sensitized.
Testing should also include additives present in each formulation
of interest. Sterile diluent vials (eg, Lily Sterile Diluent 1-ND
800) are commonly used for injection teaching and diluting
insulin. These contain additives such as metacresol and glycerin
at concentrations equivalent to insulin preparations, so they may
be helpful in skin testing.
In the case of a suspected reaction to a long-acting insulin
preparation containing protamine, sensitization to protamine
should also be excluded. Testing result should be interpreted
with care, because a significant percentage of diabetic patients
who have received neutral protamine Hagedorn insulin without
history of adverse reaction may have positive skin testing result
and/or serum-specific IgE to protamine.
530,531
Skin prick testing
for protamine may be performed at a dilution matching the
approximate concentrations contained in neutral protamine
Hagedorn insulin (350
m
g/mL, a 30-fold dilution of stock
protamine, 10 mg/mL).
532
If the insulin preparation has a stopper containing latex, skin and/
or blood testing for latex allergy may also be included in the evalu-
ation. However, many preparations of insulin are now latex-free.
Serum sicknessetype reactions to insulin are relatively rare,
and have been diagnosed primarily on the basis of clinical
symptoms. Skin biopsy has shown perivascular lymphocytic
infiltrate in some cases.
518
For evaluation of delayed-type hy-
persensitivity to insulin, intradermal skin testing result to insulin
has been reported to be positive in some patients after 24 or 48
hours.
522,532
Patch testing can also been performed for potential
contact dermatitis reactions to nickel in subcutaneous infusion
needles, acrylates in infusion catheters, adhesives in butterfly
catheters, or other additives such as parabens, phenol, and
isophane.
If timing and location of symptoms are insufficient to establish
the diagnosis, skin biopsy may be helpful to rule out systemic
dermatologic conditions such as vasculitis, psoriasis, or eczema.
Management. Initial management of suspected allergic re-
actions to insulin may include antihistamines and/corticoste-
roids, particularly for local reactions.
Changing therapy may be considered. Patients with type 2
diabetes and insulin allergy can have a trial of conventional oral
hypoglycemic medications. Liraglutide, a glucagon-like peptide-1
receptor agonist that promotes endogenous insulin secretion, has
also been used in insulin allergy.
533
TABLE LVIII. Suggested skin testing concentrations for mAbs*
Agent SPT IDT
Adalimumab†
462
40 mg/mL (full strength) 0.4 mg/mL (1/100 dilution)
Bevacizumab†
380,470
25 mg/mL (full strength) 2.5 mg/mL (1/10 dilution)z
Brentuximab vedotin
474
1.8 mg/mL 0.18 mg/mL
Certolizumab NA NA
Cetuximab†
380,470
2 mg/mL (full strength) 0.2 mg/mL (1/10 dilution)x
Etanercept†
462
50 mg/mL (full strength) 0.5 mg/mL (1/100 dilution)
Golimumab NA NA
Infliximab†
380,445
10 mg/mL (full strength) 1 mg/mL (1/10 dilution)
Obinutuzumab NA NA
Ofatumumab NA NA
Omalizumab†
508
125 mg/mL (full strength)k 0.00125 mg/mL (1/100,000 dilution)
Pertuzumab
497
1.6 mg/mL (z1/20){ 0.16 mg/mL (z1/200 dilution)x
Rituximab†
380,435
10 mg/mL (full strength) 1 mg/mL (1/10 dilution)#
Tocilizumab†
502
20 mg/mL (full strength) 20 mg/mL (full strength)
Trastuzumab
435
21 mg/mL (full strength) 2.1 mg/mL (1/10 dilution)
NA, Not available.
*All dilutions should be made in normal saline (from Picard et al).
476
†Concentrations shown to be nonirritating by testing on healthy control subjects.
zA concentration of 25 mg/mL was also shown to be nonirritating.
380
xA concentration of 0.5 mg/mL was shown to be nonirritating in 8 controls, and another group reported a concentration of 5 mg/mL to be nonirritating.
380,450
kOmalizumab should be reconstituted with normal saline to avoid false-positive results.
508
{Full-strength concentration is 30 mg/mL. Pertuzumab was tested on only 1 reactive subject, with negative results at 1.6 mg/mL (SPT) and up to 0.16 mg/mL (IDT). Higher
concentrations could be nonirritating.
#Although 1 mg/mL (IDT) is more commonly used, a maximal concentration of 10 mg/mL (full strength) for rituximab IDT is recommended by the European Network on Drug
Allergies and does not seem irritating.
380
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S61

If ongoing insulin therapy is required, changing to an alter-
native insulin preparation may be sufficient to avoid further
symptoms. In the case of immediate-type reactions, if switching
insulin preparations is not feasible or alternative therapies do not
adequately control blood sugar levels, immunotherapy in the
form of desensitization may be considered. Established desensi-
tization protocols may administer insulin as increasing in-
crements of subcutaneous doses, continuous subcutaneous
infusions delivered by insulin pumps, or (rarely) by intravenous
infusion, with either regular insulin forms or recombinant in-
sulin.
534-537
Insulin desensitization has been performed starting
with subcutaneous infusion at 1 10
3
units and doubling
every 15 to 20 minutes, up to a dose of 1 unit, then switching to
continuous infusion through the insulin pump to maintain
desensitization. Transition from regular insulin to longer-acting
forms of insulin can also be attempted in desensitization
(Table LXV). Depending on patient size and total dose of in-
sulin, blood glucose levels may be monitored before, after, and
Immediate HSR to infliximab
Pretreat with diphenhydramine (25-50 mg PO) and
acetaminophen (650 mg PO) 30 minutes before the next infusion
and depending on the severity of the reacon:
Mild
Moderate
Severe
- Start infusion at 10 mL/h
for 15 minutes
- If tolerated, increase
infusion rate to infuse
over 3 hours
d
r
am
S
e
v
e
r
M
i
ld
d
e
r
s
i
on
- Start infusion at 10 mL/h
for 15 minutes
- If tolerated, increase
infusion rate to 20 mL/h
for 15 minutes
- 40 mL/h for 15 minutes
- 80 mL/h for 15 minutes
- 100 mL/h for 15 minutes
- 125 mL/h through
compleon
- Add prednisone (50 mg
PO) x 3 over 12 hours
before infusion or
hydrocorsone (100 mg
IV) or methylprednisonole
(2-40 mg IV) 20 minutes
before infusion
- Start infusion at 10 mL/h
for 15 minutes
- If tolerated, increase
infusion rate to 20 mL/h
for 15 minutes
- 40 mL/h for 15 minutes
- 80 mL/h for 15 minutes
- 100 mL/h for 15 minutes
- 125 mL/h through
compleon
FIGURE 5. Protocol for desensitization to infliximab.
475
IV, Intravenous; PO, per os (by mouth).
TABLE LIX. Example of a 3-bag/12-step desensitization protocol that is applicable for most mAbs administered intravenously
Rituximab desensitization protocol (1000 mg)
Solution 1 250 mL of 0.040 mg/mL
Solution 2 250 mL of 0.400 mg/mL
Solution 3 250 mL of 3.969 mg/mL
Step Solution Rate (mL/h) Time (min) Volume infused per step (mL) Dose administered with this step (mg) Cumulative dose (mg)
1 1 2.0 15 0.50 0.02 0.02
2 1 5.0 15 1.25 0.05 0.07
3 1 10.0 15 2.50 0.10 0.17
4 1 20.0 15 5.00 0.20 0.37
5 2 5.0 15 1.25 0.50 0.87
6 2 10.0 15 2.50 1.00 1.87
7 2 20.0 15 5.00 2.00 3.87
8 2 40.0 15 10.00 4.00 7.87
9 3 10.0 15 2.50 9.92 17.79
10 3 20.0 15 5.00 19.84 37.63
11 3 40.0 15 10.00 39.69 77.32
12 3 80.0 175 232.50 922.68 1000.00
Total time 5 h 40 min
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S62 BROYLES ET AL

during the desensitization. For severe or refractory cases, desen-
sitization has been combined with systemic cortisosteroids or
other immune modulators such as prednisolone, rituximab,
omalizumab, or mycophenolate.
538
Delayed reactions at the injection site may be of either type III
or type IV, and skin patch testing result may be negative. Large
local reactions may respond to antihistamines or, if needed, local
injections of steroids (100 mg dexamethasone diluted in 100
units insulin and used for subcutaneous injections or continuous
infusion with insulin pump). Topical cromolyn sodium may also
be compounded as a lotion or cream for local application. If local
injections are not sufficient to control symptoms, desensitization
can also be attempted. For treatment of delayed reactions, change
in insulin preparations may also be beneficial. For type III re-
actions, successful treatment with MTX,
518
colchicine, and
6-mercaptopurine (6-MP),
539
as well as plasmapheresis,
540
have
been reported.
Conclusions. Insulin allergy is a rare, but serious condition.
Patients may be sensitized to insulin itself, to additives in insulin
preparations, or to equipment used to administer subcutaneous
doses. Evaluation begins with a history and close examination of
additives contained in the form of insulin administered
(Table LXIII). Skin testing with insulin and additives may be
helpful to evaluate immediate-type reactions, and patch testing
may be useful for delayed reactions. The first steps in manage-
ment are to identify potential alternative insulin forms and treat
symptoms with antihistamine and steroids if indicated. Large
local reactions may benefit from local steroid injection and
topical cromolyn preparation, and may improve over time. If
initial strategies are not successful in preventing reactions, sub-
cutaneous desensitization may be attempted.
Progestogens including progesterone (by Dinah
Foer, MD, Andrew MacGinnitie, MD, PhD, and
Kathleen M. Buchheit, MD)
General.
Progesterone is an endogenous steroid hormone
involved in the menstrual cycle and pregnancy. Synthetic pro-
gesterone preparations known as progestins are also used as
contraception and hormone replacement, particularly in the
setting of in vitro fertilization. Adverse reactions to both
endogenous progesterone and exogenous progestins have been
documented and were initially termed autoimmune progesterone
FIGURE 6. Suggested algorithm for the management of immediate hypersensitivity reactions to mAbs. Reactions due to cytokine release
syndrome typically occur on the first administration and wane rapidly with subsequent exposures. Symptoms usually include fever, chills,
rigors, and dyspnea but flushing, dizziness/hypotension, and gastrointestinal symptoms can also be seen. IgE-mediated and IgG-mediated
reactions generally occur after at least 1 uneventful administration although IgE-mediated reactions have been described on first exposure
to cetuximab due to preformed IgEs. IgG-mediated reactions have not been clearly demonstrated in humans, but they could account for
reactions similar to IgE-mediated hypersensitivity reactions but in which IgE antibodies cannot be demonstrated (eg, skin testing result is
negative). Grade 1 immediate hypersensitivity reactions present with symptoms that are limited to the skin (eg, flushing) or that involve a
single organ/system and that are mild (eg, mild back pain). Grade 2 hypersensitivity reactions present with symptoms that involve at least
2 organs/systems (eg, flushing and dyspnea) but without a significant drop in blood pressure or in oxygen saturation. Grade 3 hyper-
sensitivity reactions present with symptoms that typically involve at least 2 organs/systems and with a significant drop in blood pressure
(systolic 90 mm Hg and/or syncope) and/or in oxygen saturation (92%). Modified with permission from Picard et al.
476
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S63

dermatitis, but more recently, the terminology has been changed
to progestogen hypersensitivity (PH) to more accurately reflect
the pathobiology.
541,542
Hypersensitivity reactions to progester-
one are rare, with just over 100 cases reported, but with the
increased use of exogenous progestogens, PH has become more
frequently described.
543
Clinical presentation. Onset of symptoms can occur in
women any time between menarche and menopause. Endoge-
nously triggered presentations are often cyclical, because pro-
gesterone levels peak approximately 1 week before the onset of
menses.
544
In cases of PH associated with exogenous progester-
one, the timing of symptoms should correlate with progestin
TABLE LX. Desensitization protocol for etanercept and adalimumab*
Time (min) Dose (mg) Diluti on Volume administered (mL)
Etanercept
Day 1
0 0.25 1/100 1
30 0.5 1/10 0.2
60 1 1/10 0.4
90 2 1/10 0.8
120 4 Undiluted 0.16
150 4.5 Undiluted 0.18
Day 2
0 0.25 1/100 1
30 0.5 1/10 0.2
60 1 1/10 0.4
90 2 1/10 0.8
120 4 Undiluted 0.16
150 4.5 Undiluted 0.18
Day 3
0 0.5 1/100 1
30 1 1/10 0.2
60 2 1/10 0.4
90 4 1/10 0.8
120 8 Undiluted 0.16
150 16 Undiluted 0.32
180 18.5 Undiluted 0.37
Adalimumab
0 0.5 1/100 1
30 0.75 1/10 0.15
60 1.25 1/10 0.25
90 2.5 1/10 0.5
120 5 Undiluted 0.1
150 10 Undiluted 0.2
180 20 Undiluted 0.4
*Once desensitization was achieved, patients were administe red the mAb weekly to maintain desensitization (adapted from Bavbek et al
462
).
TABLE LXI. Desensitization protocol for omalizumab*
Time (min) Dose (mg) Diluti on Volume administered (mL)
0 0.0625 1/100 0.05
30 0.625 1/100 0.5
60 1.25 1/10 0.1
90 2.5 1/10 0.2
120 5 1/10 0.4
150 10 1/10 0.8
180 20 Undiluted 0.16
210 37-40† Undiluted 0.30-0.32
240 37-55† Undiluted 0.30-0.44
270 37-55† Undiluted 0.30-0.44
*The dose of omalizumab to which patients were desensitized was lower than their target dose and subsequent injections were administered weekly or biweekly.
†Depending on the total dose to be administered.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S64 BROYLES ET AL

TABLE LXII. Desensitization protocol for omalizumab (target dose ¼ 150 mg)*
Time (min) Dose (mg) Dilution Volume administered (mL)
0 1.5 12.5 mg/mL 0.12
30 3 12.5 mg/mL 0.24
60 6 12.5 mg/mL 0.48
90 12 12.5 mg/mL 0.96
120 23.75 125 mg/mL 0.19
150 48.75 125 mg/mL 0.39
180 55 125 mg/mL 0.44
*Adapted from Isabwe et al.
474
TABLE LXIII. Insulin formulations, additives, and concentrations for skin testing
522,532,933
Insulin formulation;
manufacturer Duration of action Protamine Other additives
Stock
concentration
SPT
concentration
IDT dilutions
(0.02 mL)
Insulin aspart (NovoLog);
Novo Nordisk
Rapid None Metacresol 1.72 mg/mL,
glycerin 16 mg/mL, phenol
1.5 mg/mL, zinc oxide 19.6
m
g/mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Insulin glulisine (Apidra);
Sanofi
Rapid None Metacresol 3.15 mg/mL,
tromethamine 6 mg/mL,
polysorbate-20 0.01 mg/
mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Insulin lispro (Humalog U-
100); Eli Lily
Rapid None Metacresol 3.15 mg/mL,
glycerin 16 mg/mL, zinc
oxide 19.7
m
g/mL (46
m
g/
mL for U-200)
100 IU/mL
(200 IU/mL
for U-200)
100 IU/mL 1 IU/mL
10 IU/mL
Regular insulin (Humulin R
U-100); Eli Lily
Short None Metacresol 2.5 mg/ml,
glycerin 16 mg/mL, zinc
oxide 17
m
g/100 IU
100 IU/mL
(500 IU/mL
for U-500)
100 IU/mL 1 IU/mL
10 IU/mL
Regular insulin (Novolin R);
Novo Nordisk
Regular None Metacresol 3 mg/mL, glycerol
16 mg/mL, zinc oxide 7
m
g/mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Lily Sterile Diluent (1-ND
800); Eli Lily
NA None Metacresol 1.6 mg/mL,
glycerin 16 mg/mL
NA Undiluted 1:100
1:10 (dilutions)
Insulin NPH (Novolin N);
Novo Nordisk
Intermediate Protamine sulfate
w0.35 mg/mL
Metacresol 1.5 mg/mL,
glycerol 16 mg/mL, phenol
0.65 mg/mL, zinc w33.5
µg/mL (vial) and
32.2 µg/mL (FlexPen)
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Insulin NPH (Humalin N);
Eli Lilly
Intermediate Protamine sulfate
0.35 mg/mL
Metacresol 1.6 mg/mL,
glycerin 16 mg/mL, phenol
0.65 mg/mL, zinc oxide 25
m
g/mL, glycerin 16 mg/mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Humalog 75/25; Eli Lilly Intermediate Protamine sulfate
0.28 mg/mL
Metacresol 1.76 mg/mL,
glycerin 16 mg/mL, phenol
0.715 mg/mL, zinc oxide
25
m
g/mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Novolog 70/30; Novo
Nordisk
Intermediate Protamine sulfate
0.32 mg/mL
Metacresol 1.72 mg/mL,
glycerin 16 mg/mL
(Flexpen only), phenol 1.5
mg/mL, zinc 19.6
m
g/mL,
mannitol 36.4 mg/mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Insulin detemir (Levemir);
Novo Nordisk
Intermediate/
long
None Metacresol 2.06 mg/mL,
glycerin 16 mg/mL, phenol
1.8 mg/mL, zinc 65.4
m
g/
mL
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Insulin glargine (Lantus,
Optisulin); Sanofi-Aventis
Long None Metacresol 3
m
g/mL, glycerin
1.7 mg/mL, zinc 3
m
g/mL,
polysorbate-20 2
m
g/mL*
100 IU/mL 100 IU/mL 1 IU/mL
10 IU/mL
Protamine sulfate; Fresenius
Kabi
NA 10 mg/mL Parabens 10 mg/mL 1 mg/mL
532
10 mg/mL
522,532
0.0001 mg/mL
522
IU, International unit; NPH, neutral protamine Hagedorn.
*For 10-mL vial, 3-mL cartridge has no polysorbate-20 and 3.3 higher concentrations for all other additives.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S65

exposure. Use of high-dose progestogens in women undergoing
in vitro fertilization may predispose them to the development of
PH and make it difficult for patients to undergo fertility treat-
ment.
543
Therefore, a careful history of both symptom timing
and exposures is critical to making the diagnosis of PH.
Major symptoms. PH is a multisystem disorder. It is char-
acterized by skin lesions, which include urticaria, eczema, ery-
thema multiforme, papulopustular lesions, vesiculobullous and
vesiculopustular lesions, as well as angioedema.
545-547
Non-
dermatologic hypersensitivity symptoms have also been
described, including asthma and anaphylaxis.
546,548
Both non-
dermatologic and dermatologic manifestations have been
described in the same patient.
546
Diagnosis. Diagnosis is primarily based on clinical symptoms
correlating with progestogen exposure. Skin testing for PH may
be used in the appropriate clinical context to confirm possible
cases of PH. The results must be interpreted with caution
because the sensitivity and specificity are unknown and false-
positive reactions frequently occur.
542
This may be due to the
progesterone vehicle because it is not water soluble and must be
dissolved in an oil- or ethanol-based diluent. Therefore, the skin
testing diluent should be included as a control in addition to
appropriate saline (negative) and histamine (positive) controls.
Table LXVI lists a published skin testing protocol. An uncon-
vincing history, regardless of skin test result, should prompt the
clinician to consider a broader differential diagnosis. Because
approximately half of patients with PH have positive skin test
results and there are concerns about false-positive testing results,
response of symptoms to administration of a gonadotrophin-
releasing hormone agonist has been proposed as an alternative
diagnostic strategy, at least for those with symptoms from
endogenous exposure.
542,549
Management. Management of PH varies widely on the basis
of patient’s symptoms and long-term goals. First-line treatment
includes symptom management with antihistamines and/or oral
or topical steroids as appropriate. In patients who do not expe-
rience relief with symptomatic therapies, additional therapies
such as oral contraceptive pills, tamoxifen, attenuated androgens,
gonadotropin-releasing hormone agonists, and omalizumab can
be considered.
550-553
However, these therapies may have long-
term adverse side effects limiting patient tolerance. In extreme
cases of endogenously triggered PH, oophorectomy remains a
curative option.
554
Progesterone desensitization has been demonstrated as a safe
and efficacious option for PH management. Consideration must
be given to premedication, trained staff, and clinical settings
before initiating desensitization.
543
Indications for desensitiza-
tion include need for high-dose progesterone therapy for fertility
TABLE LXIV. Short-acting insulin testing
Insulin SPT concentration (IU/mL) IDT dilutions (IU/mL)
Novolog (insulin aspart) 100 IU/mL 1 IU/mL
10 IU/mL
Humalog (lispro) 100 IU/mL 1 IU/mL
10 IU/mL
Apidra (glulisine) 100 IU/mL 1 IU/mL
10 IU/mL
Lily Sterile Diluent Undiluted 1:100 dilution
IU, International unit.
TABLE LXV. Desensitization protocol for regular/NPH insulin by subcutaneous injection*
Step Time (min) Dose administered (units) Type of insulin Cumulative dose (units)
1 0 0.01 Regular 0.01
2 30 0.02 Regular 0.03
3 60 0.05 Regular 0.08
4 90 0.1 Regular 0.18
5 120 0.2 Regular 0.38
6 150 0.5 Regular 0.88
7 180 1 Regular 1.88
8 210 2 Regular 3.88
9 240 5 Regular 8.88
10 60 min after previous dose 10 NPH 18.88
11 12 h after previous dose 10 NPH 28.88
NPH, Neutral protamine Hagedorn.
*Blood glucose by fingerstick should also be checked every 60 min during steps 1 to 10, and per routine thereafter. After the completion of this protocol, the patient should
remain on NPH 10 units twice a day. His endocrinologist can then gradually increase the insu lin dose in the outpatient setting, because this dose is likely not adequate.
TABLE LXVI. Progesterone skin testing protocol
541,542
Diagnostic modality Progesterone concentration (mg/mL)*
Skin test 50
Intradermal 0.05
0.5
5
*Diluent benzyl alcohol or olive oil.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S66 BROYLES ET AL

treatment and persistent hypersensitivity symptoms. For women
undergoing in vitro fertilization, intravaginal and intramuscular
desensitization has been used (Table LXVII, A and B).
542,543
These cases are often managed in conjunction with a repro-
ductive endocrinologist to optimize desensitization timing. Slow
oral desensitization has been proposed for patients with derma-
titis refractory to standard therapies (Table LXVII, C).
542
Sub-
sequently, patients must continuously cycle on an oral
contraceptive to maintain a steady state of progesterone to avoid
resensitization.
Glatiramer acetate (Copaxone) (by Elena Crestani, MD)
General.
Glatiramer acetate (Copaxone) is a mixture of small
polypeptides approved by the FDA as a first-line disease-modi-
fying agent in the treatment of patients with relapsing-remitting
multiple sclerosis, given its ability to decrease the frequency of
relapse and the progression of disability in affected in-
dividuals.
555
It is administered as a 1-mL subcutaneous injection,
either daily (20 mg/mL) or 3 times per week (40 mg/mL).
Major symptoms of hypersensitivity. Immediate in-
jection-site reactions are very common, occurring in up to
60% of the patients, and present with erythema, edema,
and pruritus.
556
These reactions are thought to be due to
direct mast cell activation at the site of injection causing the
release of histamine and other mediators. Other local re-
actions have also been described in the literature, thought to
be caused by either direct drug toxicity (skin necrosis,
lobular panniculitis) or immunomodulation (erythema
nodosum, urticarial vasculitis).
557-563
About 10% of patients
experience an immediate postinjection systemic reaction
characterized by various combinations of flushing, hives,
chest pain, anxiety, subjective sensation of dyspnea, and
throat constriction. These reactions can develop at any time
during treatment with glatiramer acetate, even after pro-
longed use, and their severity usually prompts discontinua-
tion of treatment, which otherwise offers a relatively benign
side-effect profile. This can be detrimental, especially for
those patients who may not be candidates for other treat-
ments. Although some of these reactions may represent
true IgE-mediated anaphylaxis, other immediate—and occa-
sionally delayed—cases are associated with negative allergy
testing and are likely due to alternative immunologic
mechanisms.
561,564-567
TABLE LXVII. Progesterone desensitization protocols
A. Intravaginal progesterone desensitization
541
Time (h) Dose (mg)*
00:00 (first day) 0.1
00:45 1
01:30 5
02:15 10
03:00 25
00:00 (next day) 50
00:45 100
01:30 100
B. Intramuscular progesterone desensitization
542
0 min 1
30 min 2
60 min 4
90 min 8
120 min 16
150 min 18.5
Total dose 50
Target daily dose Intravaginal progesterone 50-90 mg† depending on IVF protocol
C. Slow oral progestin desensitizationz
542
Day Dose (based on norethindrone component) No. of capsules 3 capsule dose per day Total daily dose
1 1.25
m
g in AM, 2.5
m
ginPM 1 1.25
m
g; 2 1.25
m
g 3.75
m
g
2 2.5
m
g in AM, 12.5
m
ginPM 2 1.25
m
g; 1 12.5
m
g15
m
g
3 12.5
m
g in AM, 25
m
ginPM 1 12.5
m
g; 2 12.5
m
g 37.5
m
g
4 37.5
m
g in AM, 37.5
m
ginPM 3 12.5
m
g; 3 12.5
m
g75
m
g
550
m
g in AM, 75
m
ginPM 1 50
m
g; 1 50
m
g þ 2 12.5
m
g 125
m
g
6 250
m
g2 125
m
g 250
m
g
7 500
m
g4 125
m
g 500
m
g
8 500
m
g4 125
m
g 500
m
g
9 1 mg 1 1mg 1mg
IVF, In vitro fertilization.
*Dosing based on target progestogen concentration.
†8% gel once daily.
zTarget dose: norethindrone 1 mg/ethinyl estradiol 0.02 mg.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S67

Diagnosis. Skin prick testing is performed using the full
concentration of 20 mg/mL of glatiramer acetate (which is
negative in nonexposed controls). If prick testing result is
negative, intradermal testing is performed using 0.00002 mg/mL
(1:1,000,000 dilution), and if still negative, 0.0002 mg/mL
(1:100,000 dilution), which is the highest nonirritating con-
centration reported in a study.
568
Previous reports indicated a
high rate of false-positive reactions in control subjects when
higher concentrations were used. Also, each 20-mg vial of gla-
tiramer acetate contains 40 mg of mannitol, which is an irritant
that could at least in part be responsible for the high frequency of
nonspecific reactions reported.
569,570
Specific IgE to glatiramer
acetate have been measured via ELISA in a few patients and levels
were found to be elevated in most, though not all, patients with
positive skin testing result.
567,569
These determinations were
performed on a research basis, because specific IgE testing is not
available commercially. Finally, BAT was reported in a series of 3
patients with systemic reactions and positive skin testing result,
and the result was positive in 2 of them. Overall, though gla-
tiramer acetate skin testing has not been validated, it appears to
be the most useful diagnostic tool to assist in the evaluation and
management of patients with a history of reactions to glatiramer
acetate to determine whether an IgE-mediated mechanism may
be involved; this could help in making a decision about future
desensitization if indicated.
Management. Successful desensitization to glatiramer acetate
in the context of immediate IgE-mediated hypersensitivity has
TABLE LXVIII. Glatiramer acetate desensitization protocol*
567
Solution Volume (mL) Concentration (mg/mL) Timing Dose (mg) Cumulative dose (mg)
1 0.5 0.000002 0:00 0.000001 0.000001
2 0.5 0.00002 0:30 0.00001 0.000011
3 0.5 0.0002 1:00 0.0001 0.000111
4 0.5 0.002 1:30 0.001 0.001111
5 0.5 0.02 2:00 0.01 0.011111
6† 0.5 0.2 2:30 0.1 0.1
7 0.5 2 3:00 1 1
8 0.2 10 3:30 2 3
8 0.3 10 4:00 3 6
9 0.2 20 4:30 4 10
*After successful desensitization, start treatment with 10 mg twice daily for 2 doses and then switch to 20 mg daily if no reactions.
†Numbers rounded at step 6.
TABLE LXIX. Rapid desensitization protocol for intravenous administration of imiglucerase (1500 IU)
578
Solution Step Rate (mL/h) Time (min) Dose administered with each step (units) Cumulative dose (units)
1 1 2.0 15 0.0300 0.0300
1 2 5.0 15 0.0750 0.1050
1 3 10.0 15 0.1500 0.2550
1 4 20.0 15 0.3000 0.5550
2 5 5.0 15 0.7500 1.3050
2 6 10.0 15 1.5000 2.8050
2 7 20.0 15 3.000 5.8050
2 8 30.0 15 4.5000 10.3050
3 9 7.0 15 10.4279 20.7329
3 10 15.0 15 22.3454 43.0783
3 11 30.0 15 44.6909 87.7691
3 12 80.01 177.75 1412.2309 1500.0000
IU, International unit.
Description: Solution 1, 0.060 U/mL; Solution 2, 0.600 U/mL; Solution 3, 5.959 U/mL.
Total administration time: 5.71 h.
Premedication: dimetindene, 4 mg administered intravenously 20 min before rapid desensitization.
TABLE LXX. Protocol for vaccine graded dose administration/desensitization*
Step Volume (mL) Dilution
1 0.05 1:10
2 0.05 Full strength
3 0.1 Full strength
4 0.15 Full strength
5 0.2 Full strength
*Adapted with permission from Kelso et al.
586
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S68 BROYLES ET AL

been described both in adult and in pediatric patients. A report
in 2010 described a desensitization protocol that was successfully
applied to 6 adult patients with a history of both immediate or
delayed systemic reactions and positive skin testing result.
571
Also, a modified, more gradual version of the protocol, which
included premedication with steroids and antihistamines, was
successfully carried out to desensitize another adult patient.
572
A
similar protocol was applied to a 51-year-old woman with
generalized urticarial and positive skin testing result to glatiramer
acetate.
569
The youngest patient ever documented to undergo
successful desensitization was a 14-year-old girl who experienced
a severe systemic reaction and was found to have positive skin
testing result; she was successfully desensitized to the full dose of
20 mg of glatiramer acetate (10 mg every 12 hours) and was
eventually transitioned to daily injections (20 mg daily) with no
further adverse reactions
568
(Table LXVIII). The same protocol
has been successfully used to desensitize another pediatric pa-
tient, and doctors were able to switch the patient to daily dosing
within 24 hours of desensitization. H
1
and H
2
blockers, anti-
leukotriene, or leukotriene receptor antagonists may be used for
pretreatment and/or treatment of breakthrough reactions.
Imiglucerase (Cerezyme) (by Joseph Zhou, MD, PhD)
General.
Imiglucerase (Cerezyme) is a highly purified human
enzyme used for long-term enzyme replacement therapy for type
1 Gaucher disease. Up to 15% of patients may experience
adverse reactions.
573
Reported adverse events include gastroin-
testinal irritation (nausea, vomiting, abdominal pain, diarrhea),
nonspecific constitutional symptoms (fatigue, fever, headache,
chills, rash, and backache), and tachycardia. Approximately 15%
of patients have developed IgG antibodies, and although more
than 90% of these patients tolerated the infusion for at least 24
to 36 months, almost half of these antibody-positive patients
later experienced symptoms suggestive of hypersensitivity.
574,575
In general, hypersensitivity reactions to imiglucerase intravenous
infusion have been reported in approximately 13.8% of patients.
IgE-mediated hypersensitivity reactions caused by imiglucerase
are not common, and anaphylaxis to imiglucerase infusion is rare
(reported in <1% of patients).
576,577
Given the detrimental consequence of anaphylaxis, patients
with symptoms suggesting hypersensitivity reaction to imiglu-
cerase need to be evaluated carefully and managed accordingly.
Skin testing and graded challenge can be used to evaluate imi-
glucerase hypersensitivity. Desensitization for continued admin-
istration of imiglucerase can be considered in patients who have
an established diagnosis of imiglucerase hypersensitivity without
appropriate alternative therapy.
Evaluation. The positive and negative predictive value of
imiglucerase skin test is unknown due to limited data; therefore,
skin testing is not recommended as a routine procedure. How-
ever, if the clinical symptoms associated with imiglucerase in-
fusions suggest possible IgE-mediated hypersensitivity, skin
testing and/or graded challenge with imiglucerase solution can be
considered. Skin testing is recommended only for those patients
who experience moderate or severe recurrent imiglucerase
infusioneassociated reactions and the reactions are suggestive of
IgE-mediated hypersensitivity such as persistent symptoms of
bronchospasm, hypotension, and/or urticaria.
Imiglucerase is reconstituted at the concentration of 200 units
in 5 mL of sterile water to make a 40 units/mL solution. This
concentration can be used for skin prick testing. For intradermal
testing, 10-fold serial dilutions can be made with 0.9% sterile
saline, and the highest nonirritating concentration for intrader-
mal testing is 4 units/mL.
573
Provider discretion can be used
regarding the least concentrated dilution at which to start testing.
For patients who experience mild adverse reactions to imi-
glucerase infusion that are not consistent with a hypersensitivity
reaction, imiglucerase may be delivered with 2- or 3-step graded
challenge protocols depending on the severity of the adverse
reaction. If a 3-step protocol is used, 1% of the total therapeutic
dose can be infused over 1 hour as the first step. If there are no
adverse reactions at the end of the infusion, the infusion rate can
be increased so that 10% of the total dose is delivered over
1 hour. The rest of the therapeutic dose (90%) can be given at
the regular rate if the patient successfully tolerates the first 2
steps. If no adverse events are noted at the end of the third-step
infusion, the patient is not likely to have imiglucerase allergy, and
the challenge is not needed in the future unless the patient de-
velops new reactions that may suggest hypersensitivity.
Management. Desensitization is recommended for patients
who h ave hypersensi tivity reactions to imiglucerase and have
no alternative treatment. An example protocol could include 5
bags (from 1 unit/mL to 0.0001 unit/mL) with 10 steps each
to deliver roughly 140 units of imiglucerase in 5 hours. Then
another 4-step infusion protocol can be used to achieve the
regular infusion rate of 100 mL/h at the final step.
573
The total
desensitization time varies depending on the dose but typically
takes 6 to 9 hours.
TABLE LXXI. Clinical presentation of immediate hypersensitivity according to the modified Ring and Messmer Scale
603,608-611
Grades Clinical signs
I Mucocutaneous signs: generalized erythema and/or extensive urticaria with or without angioedema
II Moderate multivisceral signs: mucocutaneous signs hypotension tachycardia moderate bronchospasm gastrointestinal disturbances
III Life-threatening mono or multivisceral signs: Cardiovascular collapse, tachycardia or bradycardia cardiac dysrhythmia mucocutaneous
signs severe bronchospasm gastrointestinal disturbances
IV Circulatory arrest
TABLE LXXII. Classification of LAs mostly used in the perioper-
ative setting
Amides Esters
Bupivacaine Chloroprocaine
Levobupivacaine Procaine
Lidocaine Tetracaine
Mepivacaine
Prilocaine
Ropivacaine
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S69

A few more case reports regarding successful imiglucerase
desensitization have been reported by Peroni et al
574
(a 7-step
rush protocol, total infusion time of 4 hours, final infusion rate
of 400 units/h), Erdogdu et al,
578
and Tsilochristou et al.
579
The 12-step protocol for administration of 1500 units of imi-
glucerase over 5.7 hours is presented in Table LXIX.
578
Vaccines (by Matthew Giannetti, MD)
General.
The reported incidence of any adverse effect after
vaccine administration is 11.4 per 100,000, of which fever and
injection-site reactions are most common.
580
The incidence of
IgE-mediated hypersensitivity is much lower. One retrospective
US study analyzed data from 2009 to 2011 and reported 33 of
25,173,965 cases of anaphylaxis with no deaths (1.31 cases/
million doses).
581
Another study reported an incidence of im-
mediate hypersensitivity to be 1 of 450,000 vaccines adminis-
tered. Thirty-one percent of reactions occurred after the first
administration of the vaccine, thus suggesting preexisting
sensitization.
582
Clinical manifestations. Clinical symptoms can be divided
into immediate and delayed reactions. Immediate reactions occur
minutes to hours after vaccination and may include urticaria/
angioedema, flushing, pruritus, wheezing, dyspnea, and hypo-
tension.
583
Delayed reactions occur more than 60 minutes after
vaccination and include rash, fever, and joint pain.
584
Symptoms
such as fever and injection-site edema are not considered mani-
festations of hypersensitivity and should not preclude future
doses of the vaccine.
585
Diagnosis. IgE-mediated reactions are more likely to be
directed at vaccine components than the active ingredient.
584
Causative components include gelatin, egg protein, latex, and
yeast. Therefore, skin testing should encompass the vaccine and
TABLE LXXIV. Maximum nonirritating concentrations for skin testing of opiods
79
Drug Skin prick (mg/mL) Intradermal (mg/mL)
Morphine 1 0.01
Fentanyl 0.05 0.005
Alfentanil 0.5 0.05
Sufentanil 0.005 0.0005
Remifentanil 0.05 0.005
TABLE LXXIII. Concentrations of anesthetic agents normally nonreactive during skin tests
61 1
Drugs PTs IDTs
International nonproprietary name Concentration (mg/mL) Dilution Maximal concentration (mg/mL) Dilution Maximal concentration (
m
g/mL)
Atracurium 10 1/10 1 1/1000 10
cis-Atracurium 2 Undiluted 2 1/100 20
Mivacurium 2 1/10 0.2 1/1000 2
Pancuronium 2 Undiluted 2 1/10 200
Rocuronium 10 Undiluted 10 1/200 50
Suxamethonium 50 1/5 10 1/500 100
Vecuronium 4 Undiluted 4 1/10 400
Etomidate 2 Undiluted 2 1/10 200
Midazolam 5 Undiluted 5 1/10 500
Propofol 10 Undiluted 10 1/10 1000
Ketamine 100 1/10 10 1/100 1000
Alfentanil 0.5 Undiluted 0.5 1/10 50
Fentanyl 0.05 Undiluted 0.05 1/10 5
Morphine 10 1/10 1 1/1000 10
Remifentanil 0.05 Undiluted 0.05 1/10 5
Sufentanil 0.005 Undiluted 0.005 1/10 0.5
Bupivacaine 2.5 Undiluted 2.5 1/10 250
Lidocaine 10 Undiluted 10 1/10 1000
Mepivacaine 10 Undiluted 10 1/10 1000
Ropivacaine 2 Undiluted 2 1/10 200
TABLE LXXV. Desensitization protocol for sublingual
buprenorphine*
663
Step (30 min apart) Dose (sublingual)
1 0.002
m
g
2 0.02
m
g
3 0.2
m
g
4 0.002 mg
5 0.02 mg
6 0.2 mg
7 0.6 mg
8 1.2 mg
*For target dose of 2 mg sublingual buprenorphine twice daily with cetirizine 10 mg
premedication followed by daily administration.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S70 BROYLES ET AL
all potential culprit components contained in the vaccine. When
skin testing to the vaccine itself, testing should use an identical
vaccine (same dose and manufacturer) as that which caused the
reaction.
Skin prick testing should begin with full-strength vaccine. If
the PT result is negative (with appropriate controls), proceed to
intradermal testing using 0.02 mL of a 1:100 dilution.
586
Skin
testing to gelatin, egg, latex, and yeast should be conducted in
the usual manner. If skin testing is not possible (standardized
latex and gelatin are not routinely available in the United States),
substitution with in vitro specific IgE-antibody assays is appro-
priate, though sensitivity is typically lower with specific IgE
testing.
587
If testing result is positive, the patient must be
considered allergic. Negative skin testing result virtually excludes
the possibility of an IgE antibody to the vaccine or vaccine
component.
586
Management. Patients who present with mild symptoms
that are not compatible with IgE-mediated hypersensitivity
maybeadministeredthevaccine in the usual manner. Ex-
ceptions include absolute contraindications such as Guillain-
Barré, SJS, encephalopathy, and other severe delayed
reactions.
All patients who report symptoms consistent with IgE-
mediated hypersensitivity should undergo skin testing. Since
negative skin testing virtually excludes the presence of an IgE-
mediated reaction, these patients may be given the vaccine in
the usual manner, followed by a 30-minute observation
period.
586
Alternatively, one-tenth of the vaccine may be
administered, followed by a 30-minute observation period,
then the remaining dose. Patients with positive skin testing
result are more likely allergic to the vaccine; however, patients
with positive skin testing result have also received the vaccine
uneventfully. If additional doses are required, these should be
administered usin g a graded dose protocol (Table LXX). In all
cases, appropriate medication should be available for imme-
diate treatment as necessary.
Patients with a preexisting allergy to a vaccine component
(without previous exposure to the vaccine itself) may warrant
further evaluation. Patients with gelatin allergy should undergo
skin testing to gelatin before receiving the varicella zoster, mea-
sles-mumps-rubella, or rabies vaccines.
588
Gelatin skin testing is
typically not available in the United States; testing for both
bovine and porcine gelatin specific IgE is an appropriate alter-
native. However, Kelso et al
586
describe a gelatin preparation
made by dissolving 1 teaspoon (5 g) of any sugared gelatin
powder (eg, Jell-O) in 5 mL of normal saline to create an SPT
solution, recognizing that this is not a standardized, validated,
FDA-approved method. Patients allergic to egg require evalua-
tion before receiving the yellow fever vaccine (all other vaccines
are safe in patients with egg allergy).
589,590
Patients akkergic to
yeast should be evaluated before receiving the hepatitis B and
human papillomavirus vaccines. Evaluation should include
serum-specific IgE to Saccharomyces cerevisiae (baker’s yeast) and/
or skin prick testing to S cerevisiae. Finally, a history of imme-
diate hypersensitivity to latex, neomycin, streptomycin, or
polymyxin B warrants evaluation before the administration of a
vaccine containing these compounds. Positive skin testing result
should prompt desensitization to the necessary vaccine.
Table LXX assumes a vaccine with a standard volume of 0.5
mL. Each dose should be followed by a 15-minute observation
before proceeding to the next step. After completion, the patient
should be observed for 30 minutes.
LOCAL AND GENERAL ANESTHETICS AND
OPIATES
Perioperative immediate hypersensitivity (by Pascale
Dewachter, MD, PhD, and David L. Hepner, MD, MPH)
Epidemiology.
In the early 1980s, the overall incidence of
perioperative immediate hypersensitivity was estimated to be 1 in
5,000 to 13,000 anesthetics administered in Australia, 1 in 4,600
in France, 1 in 1,250 to 5,000 in New Zealand, and 1 in 3,500
in the United Kingdom.
591-594
By the end of the 1990s, the
overall incidence of perioperative IgE-mediated anaphylaxis was
1 in 1,000 to 20,000 anesthetics administered in Australia and 1
in 13,000 in France.
595,596
Recently, over the last decade, the
combined allergic and nonallergic anaphylaxis rate with an
anesthetic was estimated to be 1 in 11,000 in Western Australia
and 1 in 10,000 in the United Kingdom.
597,598
More precisely,
an IgE-mediated mechanism has been involved in half and up to
two-thirds of the cases of perioperative immediate hypersensi-
tivity in the United States, Spain, Norway, and France.
599-602
Perioperative IgE-mediated allergy mainly occurs after anesthetic
induction and is primarily linked to neuromuscular-blocking
agents (NMBAs) and antibiotics such as
b
-lactam drugs; it may
also arise during the maintenance phase of anesthesia and agents
unrelated to anesthetics are then usually involved.
603
A female
predominance has been regularly reported for both IgE-mediated
and noneIgE-mediated hypersensitivity reactions, irrespective of
the causal agent, whereas immediate IgE-mediated drug allergy is
uncommon in children.
599-602
The incidence of perioperative
latex allergy continues to decrease.
The morbidity rate of perioperative IgE-mediated anaphylaxis
remains unknown. The latest National Audit Project (NAP6)
reported a mortality rate of 3.8% among 266 reports of
anaphylaxis from all UK National Health Service hospitals over 1
year (November 2015 to November 2016).
598
In contrast, a
previous Western Australian retrospective study (January 2000 to
December 2009) suggested that perioperative anaphylaxis mor-
tality rate is within the range of 0% to 1.4% and that the higher
rates, that is, 3% and up to 10%, reported during the last 2
decades may be an overestimate.
597,604-607
In summary, the incidence of perioperative anaphylaxis likely
remains underestimated because not all cases are investigated,
reported to the Drug Safety Monitoring Authorities, or included in
a national register. Conversely, the related mortality seems to be
lower than previously reported.
Clinical presentation. Perioperative immediate hypersensi-
tivity mainly occurs within minutes after anesthetic induction
and is primarily linked to agents administered intravenously.
603
The initial diagnosis of perioperative immediate hypersensitivity
is based on the ongoing features and their severity, as well as the
timing between the introduction of the suspected drug and the
onset of clinical symptoms. The clinical presentation of periop-
erative nonallergic hypersensitivity is usually mild or moderate
and less severe than that of IgE-mediated allergic hypersensitiv-
ity, this latter condition being mostly life-threatening.
603,608
The
care management of perioperative immediate hypersensitivity is
guided by the clinical expression of the reaction.
600,609-612
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S71
TABLE LXXVI. Desensitization protocols for AERD and non-AERD NSAID hypersensitivity reactions
Aspirin desensitization for patients with AERD*
Oral protocols
Time Aspirin dose (mg)
Two-day protocol
Dose escalation 180 min
Day 1 8:00 am 20-40†
11:00 am 40-60
2:00 pm 60-100
5:00 pm Discharge
Day 2 8:00 am 150
11:00 am 325
2:00 pm Discharge
One-day protocol
Dose escalation 90 min
8:00 am 40.5†
9:30 am 81
11:00 am 162
12:30 pm 325
2:00 pm Discharge
Intranasal ketorolac and oral aspirin protocol
Day 1 8:00 am 1.26 mg ketorolac† (1 spray in 1 nostril)
8:30 am 2.52 mg ketorolac (1 spray in each nostril)
9:00 am 5.04 mg ketorolac (2 sprays in each nostril)
9:30 am 7.56 mg ketorolac (3 sprays in each nostril)
10:30 am 60
12:00 pm 60
3:00 pm Discharge
Day 2 8:00 am 150
11:00 am 325
2:00 pm Discharge
Aspirin desensitization for patients without AERDz
Option 1: Dose escalation: Every 15-30 min until target daily dose has been tolerated
682
Day 1 0 1
15 2
30 5
45 10
60 20
75 40
90 81x
Option 2: Dose repeated every 90 min until no further reaction symptoms
682
Day 1
0 40.5
90 40.5x
180 Repeat 40.5 only if patient reactsx
Recommendations for treatment of NSAID-induced reactions
Respiratory Bronchodilators and zileuton if severe
Nasal/ocular H
1
antagonists, topical decongestants
Cutaneous H
1
antagonists and zileuton if severe
Gastrointestinal H
2
antagonists and zileuton if severe
Hypotension Intramuscular epinephrine
Laryngeal Racemic epinephrine
*Pretreatment with leukotriene receptor antagonists is strongly recommended for all AERD protocols.
†Lung function and vital signs are monitored before each dose and at the onset of a reaction. Reaction symptoms are treated for patient safety and comfort. Once symptoms
subside, typically within 3 h, the threshold dose is repeated and the dose- escalation interval resumes.
zNo medication pretreatment recommended for rapid oral protocols.
xOn subsequent days, start aspirin 81 mg daily.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S72 BROYLES ET AL
How to stratify immediate hypersensitivity. Although
the Ring and Messmer 4-step grading scale does not take into
account the pathophysiologic mechanisms involved (allergic vs
nonallergic), it is appropriate for grading the clinical severity of
drug-induced immediate hypersensitivity into 4 categories
(grades I-IV) and guiding its clinical care. This 4-step grading
scale, widely used in Europe, has been adapted as follows for the
perioperative setting (Table LXXI).
603,608-611
Grade I reactions
involve mucocutaneous signs only, whereas grade II reactions
correspond to mucocutaneous features that may be associated
with mild cardiovascular (hypotension, tachycardia) and/or res-
piratory signs. The cardinal sign of grade III reactions is car-
diovascular collapse, which may be associated with
mucocutaneous signs and bronchospasm. Grade IV reactions
present with circulatory arrest. Thus, grade I and II reactions are
not life-threatening conditions and usually of nonallergic origin
but sometimes may be IgE-mediated. However, grade III and IV
reactions are typically being referred to as anaphylaxis, that is, a
life-threatening immediate hypersensitivity more likely to be IgE-
mediated. All these grades thus require subsequent allergologic
investigation.
Major symptoms. Cardiovascular homeostasis disturbances
are the hallmark of drug-induced perioperative immediate hy-
persensitivity. These cardiovascular features are usually associated
with mucocutaneous signs and may be associated with respira-
tory symptoms. NoneIgE-mediated immediate hypersensitivity
may include mucocutaneous signs alone or hypotension associ-
ated with tachycardia and mucocutaneous signs. The most
common pattern of perioperative drug-induced IgE-mediated
allergy is consistent with cardiovascular collapse usually associ-
ated with tachycardia, or in some cases with bradycardia and
cutaneous features (generalized erythema and/or extensive urti-
caria). These cutaneous features are sometimes associated with
mucous signs (eyelid and/or lip angioedema). Particularly during
the maintenance phase of anesthesia, cutaneous signs may be
initially missed because of surgical drapes. In grade III reactions,
the cardiovascular collapse may rapidly evolve into cardiac
arrhythmia and/or circulatory arrest if not recognized and/or
treated appropriately. Cardiovascular collapse as the sole feature
or circulatory arrest may also be the inaugural event of periop-
erative drug-induced allergic anaphylaxis.
608-612
In this setting,
circulatory arrest usually presents as pulseless electrical
activity.
598,603,613,614
Mucocutaneous signs (eg, generalized erythema) are usually
present since the early stage of anaphylaxis but may be absent
before the restoration of hemodynamic parameters. Broncho-
spasm may also be present, especially in patients with poorly
controlled underlying airway hyperreactivity (eg, asthma and
chronic obstructive pulmonary disease [COPD]). In contrast,
isolated bronchospasm is never of allergic origin.
615
Gastroin-
testinal signs are usually not reported during the perioperative
setting.
603,608
Tako-Tsubo syndrome following perioperative
anaphylaxis.
Tako-Tsubo syndrome is characterized by an
acute but reversible left ventricular systolic dysfunction and
shares common features with acute coronary syndrome.
616
This
condition has been previously described under different names
including broken heart syndrome or stress cardiomyopathy or apical
ballooning syndrome. Recently, an international expert consensus
provided diagnostic criteria for the diagnosis of Tako-Tsubo
syndrome to improve its identification and stratification.
617,618
The main diagnostic criteria include the following: (1) transient
left ventricular dysfunction; (2) electrocardiographic abnormal-
ities (rare cases may exist without any electrocardiographic
changes); (3) levels of cardiac biomarkers (troponin and creatine
kinase) moderately elevated in most cases; significant elevation in
level of brain natriuretic peptide is common; (4) neurologic
disorders (eg, subarachnoid hemorrhage and stroke) and pheo-
chromocytoma may serve as triggers; and (5) emotional and/or
physical triggers may precede the syndrome.
Four major variants of Tako-Tsubo syndrome have been
described, based on the anatomic distribution of regional wall
motion abnormalities. The left ventricular apical ballooning, also
known as the typical form of Tako-Tsubo syndrome, is the most
common phenotype. Atypical phenotypes include wall motion
patterns in the basal, midventricular, and focal anatomic areas.
Tako-Tsubo syndrome following perioperative anaphylaxis has
been published. In this setting, inappropriate high doses of
exogenous epinephrine appear to be the common trigger.
619,620
The basal phenotype has been reported to be associated with
epinephrine-induced Tako-Tsubo syndrome, which is charac-
terized by a rapid onset of symptoms after epinephrine admin-
istration.
617,620
However, the contributive role of endogenous
catecholamines in response to anaphylaxis cannot be ruled
out.
621
Agents involved. Perioperative IgE-mediated anaphylaxis
usually occurs within minutes of anesthetic induction.
608
In this
clinical setting, NMBAs and antibiotics (mainly
b
-lactam agents)
are the main drugs involved.
599,601,602
Anaphylaxis may also arise
during the maintenance phase of anesthesia and is usually due to
agents unrelated to the anesthetic. These agents given during a
surgery include dyes (methylene blue, patent blue V and its
derivative isosulfan blue), colloid (modified fluid gelatin), anti-
septics (chlorhexidine, povidone iodine), iodinated contrast
agents, aprotinin (some fibrin glue products contain aprotinin),
and other biological sealants.
603,608
Ethylene oxideeinduced
anaphylaxis has also been suggested as a cause of perioperative
anaphylaxis. However, it appears that most of the reactions
attributed to ethylene oxide were due to latex.
622
Systemic
cooling has also been reported as a trigger for perioperative
anaphylaxis.
623
Finally, anaphylaxis may also occur toward the
end of the anesthetic induction or during the recovery period
after the injection of sugammadex or neostigmine used for
NMBA reversal.
624,625
Neuromuscular-blocking agents. NMBAs are quater-
nary ammonium compounds with positively charged radicals
[N
þ
(CH
3
)
3
] mimicking the quaternary nitrogen radical of
acetylcholine. This similarity in structure attracts NMBAs to
nicotinic receptors. NMBAs can be classified according to their
mechanism of action, as depolarizing and nondepolarizing
agents. Succinylcholine, a depolarizing agent, is an agonist at
acetylcholine receptors. Nondepolarizing agents are competitive
antagonists at the acetylcholine receptor and grouped according
to their chemical structure into steroidal (pancuronium,
rocuronium, vecuronium) and benzylisoquinolin (atracurium,
cis-atracurium, and mivacurium) compounds.
All NMBAs may elicit IgE-mediated allergic immediate hy-
persensitivity, which is usually a life-threatening condition, that
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S73

is, grade III reaction, and in some rare cases, consistent with an
inaugural grade IV reaction.
603,626
The substituted (tertiary as
well as quaternary) ammonium ions have been suggested to be
the allergenic determinants of NMBAs since the 1980s.
627,628
Although cross-reactivity between NMBAs is common, it is
unusual that an individual is allergic to all NMBAs.
629
Cross-
reactivity refers to the drug allergenicity and to the allergenic
profile of the patient.
A higher prevalence of serum IgE antibodies to tertiary and/or
quaternary ammonium ions among blood donors and atopic
patients was reported in Norway but not in Sweden. The only
difference in environmental chemical exposure was the use of
cough syrups containing pholcodine available only in Norway.
630
Other work demonstrated a higher prevalence of serum IgE
antibodies to tertiary and/or quaternary ammonium ions and/or
to pholcodine, morphine, and suxamethonium after pholcodine
exposure in atopic patients and in a few patients with a history of
NMBA-induced anaphylaxis.
630-632
The use of drugs containing
pholcodine was therefore questioned, because of the potential
risk for NMBA hypersensitivity.
631,632
However, no relationship
has been clinically established between these increased IgE levels
and the occurrence of NMBA allergy during a subsequent
anesthetic. The European Medicines Agency therefore stated that
the existing evidence does not support the use of pholcodine-
containing medicines as a risk for developing NMBA allergy.
633
Currently, the only known risk factor for NMBA-allergic
hypersensitivity is a previous noninvestigated immediate hyper-
sensitivity reaction that occurred during a previous anesthetic
induction conducted with an NMBA.
609-611,626
Nevertheless,
uneventful previous exposure to an NMBA does not exclude the
risk for IgE-mediated allergy during a subsequent anesthetic
induction.
608,609
In addition, NMBA allergy may occur without
previous NMBA exposure.
609,611,626,628
Finally, benzylisoquinoline NMBAs, such as atracurium and
mivacurium (both are not available in the United States), may
directly stimulate histamine release (ie, nonallergic immediate
hypersensitivity), whereas cis-atracurium does not.
Sugammadex. Sugammadex is a modified
g
-cyclodextrin
with a lipophilic core and hydrophilic periphery. It is used to
reverse neuromuscular blockade by encapsulating steroidal
NMBAs (rocuronium, vecuronium) and displacing them from
their receptors. Cyclodextrins are made up of dextrose units (
a
-,
b
-, and
g
-cyclodextrins). Sugammadex-induced immediate
allergic hypersensitivity has been reported.
625
The
g
-cyclodextrin
unit that contains 8 thiopropionate side chains may be respon-
sible for hypersensitivity reactions.
Neostigmine. Neostigmine is structurally similar to acetyl-
choline but contains a carbamate group instead of the acetyl
group. Neostigmine is an inhibitor of the enzyme acetylcholin-
esterase, which hydrolyzes the neurotransmitter acetylcholine at
synapses. Accumulation of acetylcholine at the neuromuscular
junction competitively antagonizes nondepolarizing NMBAs. A
few IgE-mediated cases of allergy to neostigmine have been
reported.
624,634
Hypnotic agents. Propofol: Propofol is an alkylphenol
derivative (2,6-di-isopropylphenol) marketed as an oil-water
emulsion using 10% soybean oil, 2.25% glycerol, and 1.2% egg
lecithin as the emulsifying agent. This intravenous induction
agent is widely used. Propofol may directly stimulate histamine
release, especially in young and/or stressed patients experiencing
TABLE LXXVII. Oral challenge protocols for acetaminophen*
Step
Acetaminophen dose (mg)
Rojas-Perez-Ezquerra et al
697
Yilmaz et al
693
1 250 10
2 500 50
3 1000-Final 250
4 500-Final
*Doses separated by 1-h intervals.
TABLE LXXVIII. Details of diagnostic testing to thienopyridines
706
Immediate hypersensitivity testing* Delayed hypersensitivity testing†
Clopidogrel 75 mg/mL Clopidogrel (20% in petroleum alba and 30% in water)
Step 1: Epicutaneous: 1/100 dilution
Step 2: Intradermal: 1/1000 dilution
Step 3: Intradermal : 1/100 dilution
Ticlopidine 6.25 mg/mL Ticlopidine (75% in water)
Step 1: Epicutaneous: 1/100 dilution
Step 2: Intradermal: 1/1000 dilution
Step 3: Intradermal: 1/100 dilution
Prasugrel 5 mg/mL Prasugrel (5% in water)
Step 1: Epicutaneous: 1/10 dilution
Step 2: Intradermal: 1/100 dilution
Step 3: Intradermal: 1/10 dilution
*Epicutanous PTs and IDTs were performed with same concentration in this series, but a further 1:10 dilution is suggested for intradermal testing.
†Patch tests read at 48 and 72 h.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S74 BROYLES ET AL
cutaneous signs (eg, localized or extensive erythema).
635
Conversely, IgE-mediated allergic hypersensitivity to propofol re-
mains extremely rare relative to its widespread use.
609-611
The few
documented propofol-induced IgE-mediated reactions have been
shown to be elicited by the isopropyl or phenol groups rather than
the lipid vehicle. A retrospective investigation of 171 anesthetic
charts from 99 patients with elevated specific IgE to egg, soy, or
peanut showed no documented IgE-mediated allergy to propo-
fol.
636
According to this study and previous reports, there is no
reason to avoid propofol in patients with allergy to egg, soy, and
peanut.
626,636
Other intravenous induction drugs: IgE-mediated allergic hy-
persensitivity to midazolam (hydrosoluble benzodiazepine) is
extremely rare relative to its widespread use.
79,610,611
Ketamine is
a hydrosoluble aryl-cyclo-alkylamine. There is no documented
report of IgE-mediated allergic hypersensitivity to this drug.
Inhaled anesthetics. Halogenated general anesthetic agents
are volatile liquids administered by inhalation of the vapor. The
chemical structures of these agents include fluorinated methyl-
ethyl-ethers (desflurane, isoflurane) and a poly-fluorinated-
isopropyl-methyl-ether (sevoflurane). There is no report of
IgE-mediated allergic hypersensitivity to these volatile
anesthetics.
Opioids. Morphine is a tertiary amine that, when insufficiently
diluted, causes nonspecific direct histamine release leading to false-
positive skin test results.
610,611
Alfentanil, fentanyl, remifentanil,
and sufentanil belong to the phenylpiperidine derivatives and have
no local effect on mast cells. No IgE-mediated allergy has been re-
ported with alfentanil, remifentanil, and sufentanil. A few cases of
immediate allergy to fentanyl have been reported, but the diagnosis
has not been proven because of methodologic issues.
637,638
Local anesthetics. Local anesthetics (LAs) belong to the
ester or amide groups (Table LXXII). Ester LAs (procaine,
chloroprocaine, and tetracaine) have a lipophilic or aromatic
group, an intermediate ester linkage, and a hydrophilic residue
with a tertiary amine. The metabolism of ester LAs is via plasma
cholinesterases. Para-aminobenzoic acid is the common metab-
olite of ester LAs inducing immediate and delayed hypersensi-
tivity reactions. Cross-reactivity is the rule among esters due to
para-aminobenzoic acid.
Amide LAs, such as lidocaine, mepivacaine, prilocaine, bupi-
vacaine, levobupivacaine, and ropivacaine, differ from esters in
that they have an intermediate amide linkage. The metabolism of
amide LAs is primarily in the liver. IgE-mediated allergy to amide
LAs is extremely rare relative to their widespread use.
609-611
Most
reported reactions are vasovagal episodes or toxic reactions from
inadvertent intravascular injection of an LA or epinephrine.
Delayed hypersensitivity to amide LAs has also been reported.
Cross-reactivity in the amide group has been established for
immediate and delayed hypersensitivity reactions.
639-641
There is
no cross-reactivity between amide and ester LAs.
Chlorhexidine. Perioperative IgE-mediated allergy to chlor-
hexidine is being increasingly recognized in Denmark and the
United Kingdom but not in France.
598,642,643
In a retrospective
(July 2004 to July 2012) single-center study, specific IgE and
skin tests (including PTs and IDT) to chlorhexidine were
completed in 228 patients investigated for suspected periopera-
tive allergic reactions.
642,643
About 10% of examined cases met
criteria for chlorhexidine allergy defined as a relevant clinical
reaction combined with 2 or more positive test results. The
highest combined estimated sensitivity and specificity was found
for specific IgE and SPT to chlorhexidine.
Latex. The incidence of perioperative latex allergy is drastically
decreasing.
600,602
This is because most hospitals now use pow-
der-free latex gloves with negligible residual protein concentra-
tions and/or nonlatex gloves.
635
In addition, the use of natural
rubber latex in medical products and/or equipment has been
reduced. Latex-induced immediate allergy is now infrequently
reported in the perioperative setting.
Diagnosis. The etiologic diagnosis of perioperative immediate
hypersensitivity is linked to a triad including clinical evidence
along with biological and allergologic results.
609-612
The inter-
pretation of the biological and allergologic assessment should
always be correlated to the careful and complete review of the
clinical history including the management care. The joint anal-
ysis of these elements helps to determine the pathomechanism
involved (allergic vs nonallergic), identify the culprit agent, and
provide subsequent and appropriate advice for further
anesthetics.
In vivo biochemical tests. Plasma histamine: Plasma his-
tamine is a preformed inflammatory mediator stored in mast cells
and basophils. An elevated concentration of plasma histamine
indicates in vivo release and is observed during both allergic and
nonallergic immediate hypersensitivity. The peak of plasma
histamine is immediate (normal <10 nmol/L), and its plasma
elimination half-life is short (w15-20 minutes). Diamine-oxi-
dase enzyme inactivates histamine in humans and shows the
highest expression in the intestine, kidney, and placenta. Because
the enzymatic activity of diamine-oxidase increases several hun-
dred-fold during gestation compared with nonpregnant in-
dividuals and plasma histamine rapidly decreases in a
concentration-dependent manner to levels below 1 ng/mL,
plasma histamine should not be measured after the first trimester
of pregnancy.
609-611,644
Plasma histamine should ideally be measured within 15 mi-
nutes after the onset of clinical features in cases of grade I re-
action, within 30 minutes after a grade II reaction, and within 2
hours in grade III and IV reactions.
609-611
Tryptase: Tryptases are neutral serine proteases stored pre-
dominantly in mast cells. In vivo, 2 major forms can be
measured. Pro-
a
tryptase reflects mast cell burden and is elevated
in mastocytosis. Mature
b
-tryptase is preferentially stored in mast
cells granules and is released during episodes of mast cell acti-
vation, such as IgE-mediated allergy. The total tryptase level
(normal <11 or 13
m
g/L) measures both forms. Tryptase can be
measured in serum or plasma. Total tryptase concentrations reach
a peak at 1 hour after the onset of the reaction and decline under
first-order kinetics (elimination half-life of w90 minutes).
609-612
Although an increase in tryptase can be measured 30 to 60
minutes after the onset of symptoms in cases of mild reactions
(eg, nonallergic immediate hypersensitivity due to histamine
release), it may not be elevated. Sampling is recommended
within 30 minutes and 2 hours in cases of grade III and IV re-
actions.
611,612
An increase in tryptase is highly suggestive of mast
cell activation, but its absence does not preclude the diagnosis of
IgE-mediated anaphylaxis. Baseline tryptase level should be ob-
tained more than 24 hours after the clinical event or when the
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S75
patient is referred for investigation and compared with acute
tryptase levels.
645,646
Histamine and tryptase concentrations correlate with the
severity of the clinical reaction.
13,611
Combined histamine
and tryptase measurements are recommended for the diag-
nosis of perioperative immediate hypersensitivity in the
United States and France.
13,611,635
The British and Scandi-
navian guidelines only recommend tryptase measure-
ment.
609,610
The collection of 24-hour histamine metabolites
(urinary methyl-histamine) is also recommended in the
United States, because it is elevated for a longer duration of
time than plasma histamine.
13,635
Its measurement has been
discontinued in Europe.
609-611
In vitro biochemical tests. Speci fic serum IgE measurement:
In vitro tests detect the presence of IgE antibodies by binding the
allergen onto a solid phase and using radioactive system detection
(radioallergosorbent test) or by binding the allergen onto a sponge
matrix using fluorescent detection (fluoroimmunoassay or CAP
system).
13,609-611
Radioallergosorbent test is now rarely used.
In vitro tests have been developed for the detection of specific
IgEs directed to the tertiary or quaternary ammonium groups of
NMBAs using a quaternary ammonium (choline chloride). They
work by coupling an analogue of choline onto a polysaccharide
support on sepharose or p-aminophenylphosphoryl-choline on
agarose, the latter being marketed only in France.
647,648
Subse-
quently, a morphine-based solid-phase IgE was proposed,
because the tertiary methyl-amino group of morphine cross-re-
acts in vitro with NMBAs.
649
In Europe, the suxamethonium-specifi c assay is currently
available among the different commercialized NMBAs. Its
sensitivity is relatively low, around 30% to 60%.
629
Specific
serum IgE measurement is also available for other drugs
including
b
-lactams (eg, ampicillin and amoxicillin and peni-
cil lins G and V), morphine, chlorhexidine, and p rotamine in
Europe and only to penicillin G and V in the United States.
Howeve r, these in vitro specificIgEassaystodrugsare
less sensitive and specificwhencomparedwithskin
testing.
13,609-611
Thus, identification of serum IgE to certa in
drugs provides possible e vidence of IgE sensitization but does
notprovebyitselfthatthedruginducedtheimmediatehy-
persensitivity reaction.
609
In addition, IgE-antibody assay is commercially available for
latex. Although skin testing has a higher sensitivity (95%-99%)
compared with IgE testing (35%-76%), a skin test reagent to latex
is not commercially available in the United States.
650
IgE-antibody
testing may be performed at the time of the reaction or later.
609-611
Basophil activation test. BAT is a sophisticated technique
using flow cytometry, which allows quantifying the ex vivo ca-
pacity of sensitized blood basophil activation. The upregulation
of certain markers (CD63 and CD203c) present on the granule
membrane is expressed on the basophil membrane on activation
with the suspected allergen. BAT might add to the etiologic
diagnosis of immediate drug hypersensitivity (eg, NMBA) and
help to identify both cross-reactive and safe alternative com-
pounds.
609-611
However, this technique is not commercially
available. The latest recommendations provided by the ENDA
and the Drug Allergy Interest Group of the EAACI stated that
BAT is recommended for diagnosing NMBA immediate hy-
persensitivity and, when available, BAT should be performed
before skin testing, especially in life-threatening reactions.
651
However, a recent study showed that combined CD63 and
CD203c markers did not increase BAT sensitivity compared
with CD203c alone in the investigation of NMBA immediate
hypersensitivity. BAT allowed identification of the culprit drug
in 80% of patients with allergy to NMBA and yielded concor-
dant cross-reactivity results in only 60% of the cases compared
with skin tests results. The authors thus conclude that BAT
combining CD63 and CD203c markers does not replace skin
testing in the assessment of NMBA allergy.
645
However, BAT is
not commercially available and the role of BAT needs to be
better defined in the diagnostic approach of NMBA-induced
immediate hypersensitivity, because skin testing is more sensitive
than in vitro tests.
609-611
Skin testing. Skin testing remains the criterion standard for
the detection of IgE-mediated allergy versus nonallergic imme-
diate hypersensitivity. All drugs to which the patient was exposed
within minutes before the clinical reaction must be skin
tested.
609-612
Investigation of anesthetics is performed by PTs
followed by IDTs using commercialized solutions undiluted or
diluted without exceeding the maximum recommended con-
centrations (ie, corresponding to the maximum nonirritant drug
concentration).
79,609-611,629
PTs may produce false-negative re-
sults, whereas IDTs are more sensitive but less specific than
PTs.
609,610
However, IDTs are more likely to trigger a systemic
allergic reaction and, thus, should be performed only if PT re-
sults are negative.
609,610
Diagnostic criteria for a positive skin test
result (including PT and IDT) and maximum recommended
concentrations have been defined in France.
611
They have been
recommended and/or adapted by others, and endorsed by
EAACI/ENDA (Table LXXIII).
79,609,610,629
Skin testing should be performed according to the patho-
mechanism of the immediate hypersensitivity reaction and
thus interpreted by readin g it within 15 to 20 minutes of the
skin test. If the PT results are negative, IDT is performed by
injecting 0.03 to 0.05 mL o f the correspondi ng drug (eg,
beginning at 1:10,000 or 1:1,000 dilution). If the IDT result is
negative, a 10-fold increased concentration is used with in-
cremental 15- to 20-minute intervals between each IDT until
the test result is posit ive or the highest nonirritant concen-
tration is achieved.
It is usua lly recommende d that skin testing be done at least
4 to 6 weeks after the c linical reaction to avoid false-negative
test results.
652
Dialysis and heavy tobacco s moking may lead
to a decr eased response due to cutane ous vasoconstriction,
whereas cutaneous reactivity may be increased in ca ses of
dermographism. Finally, skin testing can be performed at any
point during the pregnancy, especially for LAs and
NMBAs.
611
In conclusion, a suggestive clinical history with a mild reaction
without an increase in tryptase and a negative skin test result is
indicative of a nonallergic reaction, such as histamine release.
The use of preoperative H
1
-receptor antagonists reduces the
clinical effects of histamine release. Conversely, immediate hy-
persensitivity reactions requiring emergency treatment, and
associated with an increased tryptase and positive skin test results
to the suspected drug/agent, constitute evidence of an IgE-
mediated mechanism.
609-612
In this latter condition, the identi-
fied drug/agent should be avoided in the future, whereas negative
skin-tested drugs can be used for further procedures.
608,611
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S76 BROYLES ET AL
NMBAs: The sensitivity of skin tests to NMBAs in patients
having experienced NMBA anaphylaxis is greater than 95%,
and their reproducibility is excellent.
611
Testing should be
done by PTs, followed by IDTs. When skin testing result with
an NMBA is positive, investigation for cross-reactivity with
other available NMBAs should be performed to identify a safe
alternative (ie, negative skin-tested NMBAs) for further
procedures.
608,611,645,653
Sugammadex: Sugammadex may be skin tested undiluted by
PTs followed by IDTs if PT is negative (up to 1/100 dilution,
which appeared nonirritant).
625
Neostigmine: Neostigmine may be skin tested undiluted by
PTs followed by IDTs if PT is negative (up to 1/100 dilution,
which appeared nonirritant).
628
Hypnotic agents: These drugs may be skin tested undiluted by
PTs, followed by IDTs (up to 1/10 dilution) if PT results are
negative.
79,611
Opioids: Phenylpiperidines may be skin tested undiluted by
PTs followed by IDTs if PT results are negative (1/10 dilution
should not be exceeded). However, 1/10 and 1/1000
morphine dilutions are recommended for PT and IDT,
respectively.
79,611
LAs: LAs (without epinephrine) may be skin tested undiluted
by PTs followed by IDTs if PT is negative (1/10 dilution
should not be exceeded).
79,611
A protocol for subcutaneous
incremental challenge (graded challenge) involves injections of
increasing volumes of LA to which the patient has been proven
to be skin-tested negative. A single-blind saline step is done 15
to 20 minutes after the skin PT to rule out nonallergic causes.
Following this (typically 15-20 minutes), 0.1 mL, 0.5 mL, and
1 mL of undiluted subcutaneous injections of LA are used as
challenge steps. It is ideal to wait 15 to 20 minutes between
steps.
654
Chlorhexidine: Chlorhexidine (without alcohol) may be skin
tested up to 5 mg/mL by PTs followed by IDTs (up to 0.002
mg/mL) if PT is negative.
79
Latex: In contrast to the United States, in Europe, latex allergy
investigation is performed by PTs using commercial extracts.
The sensitivity of skin tests with latex is excellent.
Opioids and buprenorphine (by Parul Kothari, MD)
General
Opioids are generally used to treat both acute and
chronic pain.
The Drug Enforcement Agency classifies opi-
oids in 5 different scheduling classes on the basis of their po-
tential for abuse and addiction, which are the main concerns with
their long-term use. Structurally, opioids can be placed into the
following 4 chemical classes
655
:
Phenanthrenes, whose members include morphine, codeine,
hydromorphone, oxycodone, hydrocodone, oxymorphone,
and buprenorphine.
Benzomorphans, which include only pentazocine as a
member.
Phenylpiperidines, which include fentanyl, alfentanil, sufen-
tanil, and meperidine.
Diphenylheptanes, which include propoxyphene and
methadone.
In addition, based on their interaction with the
m
,
k
, and
d
receptors, opioids can be classified as agonists (eg, morphine),
partial agonists (eg, buprenorphine), or antagonists (eg,
naloxone), with the latter used to treat opioid overdose.
Major symptoms of hyperse nsitivity. True type I im-
mediate hypersensitivity reactions to opioids are rare and are
limited to case reports in the literature.
656-658
Most adverse re-
actions to opioids are side effects, often due to nonspecific, direct
release of mast cell mediators through interactions with the mast
cell opioid receptor. However, it is often difficult to distinguish
between these 2 possibilities because their clinical manifestations
can overlap. Both can cause pruritus, flushing, urticaria, nausea,
vomiting, bronchospasm, and hypotension. DHRs to opioids
have also been reported.
659,660
Diagnosis. Although some case reports have demonstrated the
presence of specific IgE via skin or serum testing, currently there
is no validated test for diagnosing an immediate hypersensitivity
reaction.
656-658
Skin testing with narcotics can lead to nonspe-
cific release of mast cell mediators, thereby eliciting a wheal-and-
flare response even in the absence of specific IgE.
661
As such,
distinguishing between immune- and nonimmune-mediated re-
actions is based on a careful history as well as physical exami-
nation findings at the time of the reaction. Nonirritating
concentrations for skin testing that have been reported are pre-
sented in Table LXXIV.
79
Although ACD has been reported for several different opioids,
it appears to be most commonly associated with transdermal
buprenorphine.
659,660
In these small case series, the diagnosis was
confirmed with patch testing, and the ability to tolerate other
opioids (oral and/or transdermal) was demonstrated. In 1 case,
oral buprenorphine was given without any adverse reaction.
Management. Prevention of future reactions will depend on
the underlying mechanism. Nonspecific adverse reactions, a class
effect, can be inhibited by pretreating with antihistamines and/or
steroids, using lower doses, or using opioids with less histamine-
releasing properties, such as fentanyl.
662
However, for immune-
mediated hypersensitivity reactions, strict avoidance of the
culprit and metabolites is recommended. As such, because co-
deine is metabolized to morphine, patients who are able to
tolerate codeine but react to morphine are unlikely to have a true
allergy but those with evidence of an IgE-mediated allergic re-
action to morphine should also avoid codeine.
For patients with evidence of an immediate or delayed hy-
persensitivity to an opioid, currently data to determine the risk of
cross-reactivity within and across different structural classes are
limited. Case reports and series have shown that most patients
with an allergic reaction are able to tolerate at least 1 other opioid
but too few patients have been studied to make accurate pre-
dictions. Thus, if a patient requires treatment with a narcotic, it
is recommended to use one that has been tolerated previously. If
that information is not known, one should determine which
alternative agent(s) could be safely administered by performing
an oral challenge in a monitored setting. There are currently no
standardized desensitization protocols for narcotic analgesics, but
a desensitization protocol to sublingual buprenorphine has been
reported for a noneIgE-mediated reaction (Table LXXV).
663
The serial dilutions for desensitization were made by grounding
and suspending buprenorphine sublingual tablets (2 mg). Given
the lack of data on the stability of buprenorphine in solution,
dilutions were prepared close to the time of administration to
minimize any possible loss of potency.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S77
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
Aspirin (by Katherine N. Cah ill, MD, and Ari J. Fried,
MD)
General.
Aspirin and other nonsteroidal anti-inflammatory drugs
(NSAIDs) are unified by their ability to inhibit cyclooxygenase
(COX)-1. Any inhibitor of COX-1 can cause acute or delayed
hypersensitivity reactions. Aspirin-induced reactions are generally
the result of altered eicosanoid pathways following COX-1 inhibi-
tion. The ease of access to NSAIDs and their clinical utility make
them one of the leading causes of hypersensitivity reactions. The
reported prevalence of hypersensitivity reaction to aspirin and other
COX-1 inhibitors in children is 0.3% and in adults is 1.9%; the
prevalence among patients with asthma is an estimated 5% in
children and 7.2% in adults.
664-667
True NSAID hypersensitivity
needs to be distinguished from an adverse reaction or intolerance to
an NSAID such as tinnitus or gastrointestinal bleeding.
Major symptoms of hypersensitivity. Cross-reacting
COX-1 inhibitor reactions include (1) aspirin-exacerbated res-
piratory disease (AERD), (2) NSAID-exacerbated cutaneous
disease, and (3) NSAID-induced urticaria and/or angioedema.
17
Acute NSAID hypersensitivity reactions that do not cross-react
are known as selective NSAID-induced urticaria, angioedema,
and/or anaphylaxis. Selective NSAID-induced delayed reactions
such as mild maculopapular exanthems, SJS, TEN, fixed drug
eruptions, DRESS, pneumonitis, aseptic meningitis, and
nephritis are much less common than acute reactions
(Table LXXVI).
668
Both acute and delayed reactions have been
reported in children and adults.
669
AERD is unique among the NSAID-exacerbated reactions
with the associated comorbidities of asthma and nasal polyposis.
NSAID-exacerbated reactions in AERD include nasal conges-
tion, rhinorrhea, postnasal drip, ocular injection, ocular and
oropharyngeal pruritus, and bronchospasm within 15 to 180
minutes of NSAID exposure. Less frequently, angioedema, ur-
ticaria, macular eruptions, abdominal pain, nausea, vomiting,
diarrhea, and hypotension have been reported. AERD is gener-
ally considered an adult-onset disease, but AERD has been re-
ported to develop as early as at age 8 years.
670
Diagnosis. The diagnostic workup for identification of
NSAID hypersensitivity is the same for children and adults.
Distinguishing selective COX inhibitor hypersensitivity from
cross-reactive hypersensitivity is crucial to determine the best
strategy for further management. The evaluation has to start with
a careful analysis of the history including symptom pattern and
time course of the reaction.
Oral aspirin challenge is the criterion standard for the diagnosis
of AERD.
671
NSAID-exacerbated cutaneous disease (NECD)
patients have chronic urticaria and a history of an acute flare of
urticaria and/or angioedema from NSAID, while patients with
NSAID–induced urticaria and/or angioedema (NIUA) have had
acute urticaria and/or angioedema from NSAID, and do not have
an associated chronic respiratory or cutaneous condition. Patients
with either NECD or NIUA exhibit cross-reaction to structurally
unrelated COX-1 inhibitors—which should be avoided. Chal-
lenge procedures are commonly performed to aspirin in these
patients when aspirin 81 mg daily is required for cardioprotection.
Desensitization is not recommended in the setting of NECD but
can be accomplished in patients with NIUA. For patients with a
history suggesting selective NSAID-induced urticaria, angioedema,
and/or anaphylaxis, a challenge to another COX-1 inhibitor can
be performed to rule out a cross-reacting NSAID hypersensitiv-
ity.
17,672
The role of skin testing or basophil activation testing to
NSAIDs has not been validated and is not recommended. Serum-
specific IgE has been reported in a case series of pyrazolone-
induced anaphylaxis, a class of NSAIDs no longer available in the
United States.
673
The detection of serum-specific IgEs for other
NSAIDs has not been reported. In the setting of severe delayed
reactions, reexposure to the culprit agent is not recommended and
exposure to another member of the NSAID class can be consid-
ered if medically necessary. With rare exception, selective COX-2
inhibitors are tolerated by patients with a history of COX-1 hy-
persensitivity reactions, but reactions to selective COX-2 in-
hibitors in patients with a history of COX-1 inhibitor
hypersensitivity have been reported; as a precaution it may be
appropriate to administer the initial dose under observation based
on clinical circumstances.
674
Management. Although challenge protocols confirm the diag-
nosis and subtype of NSAID hypersensitivity, desensitization pro-
tocols provide a means to allow the patient to safely tolerate daily
aspirin (Table LXXVI).
675
Aspirin challenge or desensitization
followed by daily aspirin therapy is indicated for adult and pediatric
patients with AERD who require revision polypectomies or
frequent or daily corticosteroid therapy. Any patient with a history
of an acute NSAID hypersensitivity reaction with a medical indi-
cation (cardiovascular or rheumatologic disease) for aspirin or other
COX-1 inhibitor should be offered desensitization.
676
Before
initiating a challenge or desensitization protocol, it is recommended
that FEV
1
be greater than 60% predicted and at least 1.5 L. Use of
a leukotriene receptor antagonist in advance of the desensitization
in patients with AERD decreases the severity of bronchospasm.
677
In a small subset of patients with AERD, the use of leukotriene
receptor antagonists will completely mask the symptoms of a re-
action. However, it is generally accepted that the added safety
benefit from their use outweighs this risk. Inhaled or oral cortico-
steroids and long-acting bronchodilators should be continued and
asthma should be optimized at the time of desensitization, with oral
steroids added if necessary. Oral antihistamines and decongestants
arediscontinued48hoursbeforeandshort-acting
b
-agonists the
morning of desensitization to avoid masking a clinical reaction.
Challenge/desensitization protocols for AERD can take place in the
outpatient setting, including for pediatric cases, with the exception
of patients with recent myocardial infarction, continuous
b
-blocker
therapy, or uncontrolled asthma.
678
A standard oral aspirin desensitization protocol for AERD
begins with a dose of 40 mg of aspirin, with dose escalation every
90 to 180 minutes, as outlined in Table LXXVI. At the onset of
reaction symptoms, the patient is monitored and symptomatic
treatment with
b
-agonists, antihistamines, nasal decongestants,
and 5-lipooxygenase inhibitors can be used to ensure patient
safety and comfort. Shorter oral protocols have been reported in
the literature.
679
Alternatively, a modified protocol using intra-
nasal ketolorac is available that reduces extrapulmonary reactions
during desensitization.
680
Once a patient has tolerated 325 mg of
aspirin without evidence of symptoms, they can increase their
dose of aspirin to the recommended treatment dose of 650 mg
twice daily at home. If aspirin is discontinued for more than 48
hours, the desensitized state can be lost and repeat desensitization
is recommended. In the event of a planned surgical procedure
during which aspirin should be avoided, the daily aspirin dose
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S78 BROYLES ET AL
can be decreased to 81 mg leading up to the procedure, held the
day before and the morning of the procedure, and immediately
resumed after the procedure is completed.
For other non-AERD reactions to NSAIDs, challenge pro-
tocols are published.
675
In clinical practice, there is less consensus
about the dose and timing intervals for challenge/desensitization
due to the wide range of clinical history, reaction severity, and
subtype of NSAID hypersensitivity encountered. Aspirin is the
preferred agent in a desensitization protocol for non-AERD
NSAID hypersensitivity because there are no verified reports of
anaphylaxis from aspirin.
681
For those with non-AERD NSAID
hypersensitivity and a clinical indication for daily aspirin use such
as after cardiac stent placement, aspirin challenge or desensiti-
zation using 1 of 2 rapid protocols has demonstrated success.
682
Both protocols reach the established antiplatelet threshold of 81
mg within 2 to 3 hours (Table LXXVI).
Acetaminophen (by Samantha Minnicozzi, MD)
Acetaminophen is one of the most commonly used drugs in the
world. It is a medication that is generally well tolerated, and it is
frequently used as an alternative agent for patients with aspirin
hypersensitivity.
683
Hypersensitivity reactions are exceedingly rare,
with limited data and reports of suspected IgE-mediated reactions.
There are numerous case reports and some case series describing
anaphylaxis or anaphylactoid reactions to acetaminophen.
683-696
Acetaminophen is classified as an NSAID, but it is a weak
cyclooxygenase inhibitor and does not provide any anti-inflam-
matory effects.
683
However, a number of patients who are aspirin
intolerant can also be intolerant of acetaminophen, and some
acetaminophen-specific reactions can also be either dependent on
or independent of dose.
684,689,691,692
Major symptoms of hypersensitivity. In a review of
acetaminophen hypersensitivity reactions reported over a 4-year
period to a private allergy clinic in France, 84 patients were
identified.
683
Of these 84 patients, 13 were considered identified
to have an allergy based on history and oral challenge testing.
Their symptoms consisted of maculopapular eruptions, urticaria,
bronchospasm, rhinitis, and laryngeal edema.
683
Other case re-
ports highlight patients who experience similar symptoms but
also have hypotension, vomiting, and/or diarrhea.
686,690,694,697
In addition to immediate hypersensitivity reactions, case re-
ports in the literature have described acetaminophen’s association
with SJS reactions.
698
Diagnosis. The validity of skin testing to acetaminophen is
unknown. However, in case reports citing skin prick and intra-
dermal testing at various concentrations, all control subjects have
been negative.
685,687,688,692,696,697
Of the skin testing protocols
proposed, many are not feasible because the sterile forms
required for intradermal testing do not exist in many countries.
Currently, the only intravenous formulation of acetaminophen is
a single concentration of 10 mg/mL. This only allows for in-
tradermal testing to occur with dilutions of this formulation. The
protocol described by Rojas-Perez-Ezquerra et al
697
uses the
intravenous formulation of acetaminophen with a concentration
of 10 mg/mL for skin prick testing, and a dilution of 1 mg/mL as
well as the undiluted 10 mg/mL for intradermal testing. How-
ever, patients and control subjects in that study tested negative
on skin prick and intradermal testing.
Other reviews published on acetaminophen allergy use solely
SPTs up to concentrations of 200 mg/mL despite evidence
elsewhere demonstrating that concentrations more than 10 mg/
mL can be known irritants leading to false positives for
drugs.
688,692,696
Management. Of the studies identifying patients with a
history concerning for acetaminophen hypersensitivity, the cur-
rent recommendations for definitive evidence of a reaction are
oral-based challenge tests.
696
This is because of the relatively
infrequent occurrence of hypersensitivity reactions associated
with acetaminophen use.
683,688,689,695
Table LXXVII highlights
the common methods of graded challenges performed for both
suspicion of anaphylaxis and NSAID-related reactions.
693,696
A
recent meta-analysis including 259 patients who underwent oral
challenges to acetaminophen for diagnostic confirmation or
exclusion estimates the prevalence of true acetaminophen hy-
persensitivity to be 10.1% in adults and 10.2% in pediatric
patients.
699
ANTICOAGULANTS AND COA GULATION
FACTORS
Clopidogrel and antiplatelet agents (by Kimberly
Blumenthal, MD)
General.
Clopidogrel (Plavix) is a selective, irreversible inhib-
itor of ADP-induced platelet aggregation for oral use. It belongs
to the class of second-generation thienopyridine antiplatelet
agents. Within ADP2Y12 platelet receptor inhibitors, there are
thienopyridines (clopidogrel, prasugrel, and ticlopidine) or
cyclopentyl-triazolo-pyrimidines (ticagrelor and cangrelor). Clo-
pidogrel is the standard of care for patients who have undergone
coronary stenting, especially in drug-eluting stents. Additional
indications include acute management of myocardial infarctions,
peripheral artery disease, or cerebral vascular accident. The
incidence of hypersensitivity reactions to clopidogrel have been
reported to range from 1% to 6% of exposures, with most es-
timates ranging from 1% to 3%.
700-702
Major symptom s. The most common hypersensitivity re-
action to clopidogrel is rash, commonly macular, morbilli-
form, or diffuse and erythematous, beginning about 5 days
into treatment.
703,704
In addition to these likely T-
cellemediated drug eruptions, IgE-mediated reactions
comprise about 5% to 7% of clopidogrel hypersensitivity re-
actions.
705,706
In 1 case series, patients with urticaria repre-
sented 17% of reactions to clopidogrel.
707
Worldwide
postmarketing pharmaceutical experience reports that
anaphylactic reactions occur in fewer tha n 1% of cases.
701
Other reactions including systemic hypersensitivity s yndromes
have been described, including a serum sickness elike reaction,
fixeddrugeruption,andSJS.
708
Current literature review does
not describe DRESS syndrome, TEN, or acute interstitial
nephritis attributed to clopidogrel use.
Diagnosis. There is no validated skin testing or patch testing
to clopidogrel. However, diagnostic testing could follow the
procedures of the largest case series (42 patients) evaluated with
both immediate hypersensitivity testing and patch testing after a
history suggestive of hypersensitivity to clopidogrel
(Table LXXVIII).
706
Of the 42 patients tested, most had a his-
tory consistent with a delayed rash. Although none were positive
by epicutaneous prick, 3 patients—all with previous symptoms
suggestive of an IgE-mediated reaction—were positive on
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S79
intradermal testing. Most patients, 34 of 42 or 81%, had positive
patch testing result to clopidogrel.
706
Caveats include that there
were no documented controls and there are no data to inform a
nonirritating skin testing concentration for immediate hyper-
sensitivity skin testing. In addition, the utility of testing for
cutaneous eruptions that are not IgE-mediated is unknown, but
can be helpful for some cutaneous eruptions, including mac-
ulopapular eruptions, AGEP, and fixed drug eruptions.
13,51,709-
711
Management
Alternative agents.
Alternative agents could include
another thienopyridine (ticlopidine or prasugrel), ticagrelor,
warfarin, or cilostazol.
Ticlopidine is as effective as clopidogrel. It is not first-line
therapy because of an unfavorable side-effect profile, including
serious adverse reactions of neutropenia and thrombotic
thrombocytopenic purpura (in 2%). There is concern about
cross-reactivity between clopidogrel and ticlopidine because the
structure differs only by an addition of a caboxymethyl group
and they have shared metabolites in vitro ( Figure 7). Cross-
reactivity determined by patch-test result was found in 24% of
patients.
706
Given available data to date, if ticlopidine were to be
used in a patient with a history of allergy to clopidogrel, an oral
challenge would be advised (Table LXXIX).
Prasugrel is the most potent of the thienopryridines, but it is
contraindicated in patients with previous cerebrovascular acci-
dent, age more than 75 years, or weight less than 60 kg. There is
concern regarding cross-reactivity to clopidogrel, because they are
structurally similar (Figure 7 ). No data currently exist on cross-
reactivity between clopidogrel and prasugrel, because the clinical
trials for prasugrel excluded patients with a history of allergy to
ticlopidine or clopidogrel. One study demonstrated a 17% cross-
reactivity with prasugrel using patch testing, and there are reports
of patients with a history of allergy to clopidogrel who subse-
quently tolerated prasugrel.
706,712-714
Given available allergy
data, if prasugrel were to be used in a patient with a history of
allergy to clopidogrel, or vice versa, an oral challenge would be
recommended (Table LXXIX).
Ticagrelor, which is a reversible P2Y12 inhibitor and a strong
antiplatelet, is completely structurally dissimilar from clopidog-
rel. Although a previous report hypothesized cross-reactivity,
review of the case and symptoms reported more likely reflected a
reaction to the clopidogrel and stronger evidence supports that
ticagrelor can be safely administered to patients with clopidogrel
hypersensitivity.
715-717
Oral challenge. An oral graded challenge to clopidogrel
would be appropriate if the reaction (1) was unlikely to have
been caused by clopidogrel, (2) was not IgE-mediated/serious/
life-threatening and the benefits of clopidogrel use outweighed
the risk of reaction, and (3) led to initiation of a potentially cross-
reactive drug (eg, ticlopidine).
Desensitization. Desensitization protocols for clopidogrel
hypersensitivity are safe and successful, and have been used both
for IgE-mediated reactions and for delayed cutaneous reactions.
Contraindications to desensitization include severe T-
cellemediated reactions such as DRESS, TEN, or SJS. In a study
of 24 patients who underwent a desensitization procedure, 100%
of the patients had successful desensitization and all patients were
taking clopidogrel at the 6-month follow-up. Most patients
(83%) did not have reactions during the desensitization pro-
cedure.
707
Published desensitization procedures range from 2 to
8 hours (Table LXXX).
718
The 2-hour or 7-hour desensitization
procedures are recommended on the basis of severity of the al-
lergy history, considering both the type and severity of the re-
action, as well as considering comorbidities and acuity of the
patient’s illness. Recently, outpatient multiday protocols have
been used for patients with clopidogrel hypersensitivity (largely
rash, but 1 patient had angioedema) with success.
719
One
disadvantage of using a desensitization protocol for clopidogrel
hypersensitivity, particularly after stent placement, is that it re-
quires initial cessation of clopidogrel to allow for a washout
period and symptom resolution. However, patients may be
treated with an alternative agent (eg, ticlopidine) during this
period.
Drug continuation with treatment with steroids and
antihistamines.
Because cardiology data show a significant
risk (25%-30%) of thrombosis with interruption of antiplatelet
therapy, some groups have used a “treating though” strategy for
cutaneous clopidogrel hypersensitivity reactions
(Table LXXXI).
720
Sixty-two patients with cutaneous reactions
(both urticarial and nonurticarial rashes) to clopidogrel were
treated with a 30-mg twice-daily tapering prednisone course and
benadryl 25 to 50 mg Q6-8PRN without drug stoppage. This
approach was successful in 98% of patients, with the rashes
resolving in about 5 days and all patients had uninterrupted dual
platelet therapy. Among their 62 patients, this approach failed in
1 patient who developed angioedema and required hospitaliza-
tion.
706
Among 25 patients with delayed rashes who were treated
with steroids (methylprednisolone) and antihistamines (fex-
ofenadine 180 mg every day with 25-50 mg diphenhydramine
QHS) for a mean of 10 8 days without interruption of clo-
pidogrel, 22 of 25 (88%) patients had no adverse effects/events
in long-term follow-up, and all completed clopidogrel therapy
according to the America Heart Association guidelines.
705
The
mean duration of steroid treatment was 10 days. Two patients
had recurrent symptoms, treated with more corticosteroids (18
days starting with prednisone 60 mg), an antileukotriene
(montelukast 10 mg), and antihistamines. In sum, 3 patients
failed this therapy, with 1 patient each having angioedema, a
desquamating rash, and intolerable pruritus. Another group used
prednisone 30 mg twice a day with cetirizine 10 mg daily with
success.
721
Given these encouraging data, this strategy is
reasonable for benign, maculopapular, or erythematous eruptions
that are likely to be T-cellemediated and without organ
involvement. However, this strategy is not recommended in IgE-
mediated reactions, especially those involving organ systems
beyond the skin, where desensitization is indicated, or rashes
suggestive of a severe T-cellemediated reaction. Caution is also
warranted when using this approach for cutaneous reactions
suggestive of IgE (eg, urticaria) given that these studies demon-
strating this approach to urticarial rashes are not robust enough
to determine the safety of this approach.
Heparin and protamine (by Cosby Stone Jr, MD,
MPH, and Allison Norton, MD)
General.
Heparin is a widely used anticoagulating agent for the
active treatment and prophylaxis of thrombosis in at-risk pa-
tients. Side effects of heparin are overall quite rare considering
the frequency of its use in hospitalized patients for the prevention
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S80 BROYLES ET AL
of deep venous thrombosis. Most reports of hypersensitivity to
heparin are DHRs, which can affect up to 7.5% of patients
treated, and heparin-induced thrombocytopenia (HIT).
722-724
There are, however, rare reports of immediate hypersensitivity
reactions.
725,726
In the past, when heparin products were
contaminated with overly sulfated chondroitin sulfates, non-
eIgE-mediated anaphylaxis was reported via the kinin-kalikrein
pathway through the production of bradykinin, as well as C3a
and C5a anaphylatoxins.
727
In addition, immediate hypersensi-
tivity to protamine, the main reversal agent for heparin-mediated
anticoagulation, has occurred, and can easily be mistaken for an
allergy to heparin or insulin.
728,729
Heparins are sulfated carbohydrates of the glycosamino-
glycan family first purified from pig intestines with the
property of being naturally occurring activators of anti-
thrombin III, and they achieve their anticoagulation effects
by antithrombin IIIemediated inactivation of thrombin.
730
Unfractionated heparin (UFH) can include heparins of
various molecular weights and lengths, typically averaging 14
to 18 kDa.
722,730
Low-molecular-weight heparins (LMWHs)
have been modified by fractionation or depolymerizaton to
provide a purified product, in which at least 60% of chains
are less than 8 kDa in length, to reduce the biological
unpredictability of UFH. Heparinoids are synthetic mole-
cules designed to mimic the binding site of heparin to
antithrombin III (Table LXXXII).
722
Because of their strong negative charge, UFH and LMWHs
can be inactivated by the highly cationic peptide protamine, a
compound originally derived from salmon spermatozoa. Prot-
amine has itself been shown to cause IgE-mediated hypersensi-
tivity reactions, especially in patients previously exposed to
protamine-containing insulins.
13,531,728,731
Fish allergy and va-
sectomy have previously been alleged as risk factors for protamine
reaction, but there is no substantiated evidence to support this
claim.
732,733
Cross-reactivity. Wide ranges of cross-reactivity have been
reported to occur in LMWHs in DHR, but fondaparinux and
danaparoid are generally well tolerated.
722
Hypersensitivity to
UFH is reported to be frequently cross-reactive with LMWHs
and heparinoids.
723,726
Hypersensitivity to heparin is not cross-reactive with struc-
turally distinct anticoagulants such as hirudins or factor Xa in-
hibitors, and these are often used as alternative agents.
725,734
Major symptoms of hypersens itivity. Heparins have
been demonstrated most commonly to cause DHR, of which the
most typical feature is an itching, eczematous plaque, or mac-
ulopapular eruption that first appears around sites of injection
after 7 to 10 days of continuous initial treatment, and can appear
more rapidly on subsequent exposures.
723,726,730,735,736
Immediate-type hypersensitivity to heparins can present with
palmoplantar pruritus, urticaria, conjunctivitis, bronchospasm,
and anaphylaxis, and are only rarely reported.
722,726,730,737
Hy-
potension, angioedema, and swelling of the larynx have devel-
oped in patients after receiving heparin products contaminated
with oversulfated chondroitin sulfates.
727
Mild thrombocytopenia is noted in the setting of UFH use in
about 30% to 50% of critically ill patients, but severe HIT via
IgG specific for large complexes of heparin bound to platelet
factor 4 occurs in around 1%.
722,738
HIT usually occurs after 5
days of treatment with either UFH or LMWHs, and, in severe
cases, can present with profound thrombocytopenia, thrombosis,
or cutaneous necrosis.
13,730,739,740
Immediate reactions can
occur on the first dose for previously sensitized patients with
HIT, with symptoms including fever, flushing, dyspnea, mental
status change, and hypertension.
722
Immediate hypersensitivity to protamine has been reported
with urticaria, hypotension, and anaphylaxis, via IgE- and none
IgE-mediated mechanisms.
13,530,531,728,731,741
Dose-dependent
hypotension after rapid infusion of protamine is most likely
triggered by nonspecific histamine release.
13
Protamine is a rare
cause of intraoperative anaphylaxis and is most often reported
during cardiac surgeries when quick reversal of heparin anti-
coagulation is required.
531
Cases of delayed hypersensitivity to protamine with
erythematous plaques at injection sites can occur to protamine-
containing insulin, with 1 report including eosinophilia and renal
dysfunction.
742,743
Diagnosis. Nonirritating concentrations for the evaluation of
immediate hypersensitivity to heparins and heparinoids have
been reported and shown to be useful in clinical decision
FIGURE 7. Biochemical structures of the thienopyridines.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S81

making.
79,726
Skin testing is generally performed via PT with
commercially available undiluted product. IDTs with 1:10 and
1:100 dilution may be useful, though lower concentrations are
associated with lower sensitivity.
79
The European concentration
for heparin is 25,000 units/mL for prick and 2,500 units/mL for
IDT, but the highest US concentration is 10,000 units/mL, so
PT with 10,000 units/mL and IDT with 1,000 units/mL is
recommended. In clinical experience, because cross-reactivity
between UFH, LMWHs, and heparinoids can occur, it is rec-
ommended that a panel of all these products be used during
testing.
79
Diagnosis of delayed hypersensitivity to heparins has been
reported to be more sensitive when using delayed intradermal
testing at 1:10 dilution compared with patch testing. These are
typically read 2 to 7 days after placement.
79,723,744
Testing for IgG antibodies to platelet factor 4 is the mainstay
of early diagnosis for HIT when it is suspected clinically, with a
sensitivity greater than 90% and quick turnaround times.
738
Serotonin release assays remain the criterion standard for HIT
diagnosis in terms of specificity, but they are limited by avail-
ability, specialized equipment, and longer time to obtain
results.
738
Immediate hypersensitivity s kin testing and serum-specific
IgE testing to protamine have been reported in the literature,
but have not been well studied and recommended testing di-
lutions vary widely.
530,728,745
Ebo et al
629
suggest using a
maximum undiluted protamine concentration of 50 mg/mL
for skin prick testing and 50
m
g/mL maximum concentration
for intradermal testing. However, a case series using 53 con-
trols suggested that intradermal testing at 30
m
g/mL may cause
nonspecific histamine release. They suggest skin prick testing
at concentrations of 300 to 330
m
g/mLand0.03to30
m
g/mL
for intradermal testing.
532
Management. Management of heparin sensitivity should
focus on choosing an alternative nonecross-reactive agent or
desensitization if there is no alternative. Patients with hyper-
sensitivity to UFH are often cross-sensitized to LMWHs, and
additionally may be sensitized to heparinoids.
722
Given that the
negative predictive value of heparin and protamine skin testing
remains unknown, a high suspicion of clinical reactivity with
negative testing still warrants desensitization or if feasible, se-
lection of an alternative agent. Generally, patients with both
immediate and delayed hypersensitivity can tolerate the non-
structurally related hirudins, factor Xa inhibitors, but rare pa-
tients with sensitization to structurally dissimilar anticoagulants
have been reported.
722
TABLE LXXIX. Ticlopodine test dose protocol (graded oral challenge)
Step 1
1
/
4
of a pill/dose, observe for 60 min
Step 2 1 pill/full dose, observe for 60 min
TABLE LXXX. Desensitization protocols for clopidogrel
718
Time (h) Dose Concentration mL
Seven-hour protocol
0:00 0.005 0.5 mg/mL 0.01
0:30 0.01 0.02
1:00 0.02 0.04
1:30 0.04 0.08
2:00 0.08 0.16
2:30 0.16 0.32
3:00 0.3 0.6
3:30 0.6 1.2
4:00 1.2 5 mg/mL 0.24
4:30 2.5 0.5
5:00 5 1
5:30 10 2
6:00 20 4
6:30 40 8
7:00 75 75-mg tablet 1 tablet
Two-hour protocol
0:00 0.02 0.5 mg/mL 0.04
0:15 0.05 0.1
0:30 0.15 0.3
0:45 0.5 1
1:00 1.5 5 mg/mL 0.3
1:15 5 1
1:30 15 3
1:45 45 9
2:00 75 75 mg 1 tablet
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S82 BROYLES ET AL

Several case reports have described successful heparin desen-
sitization for immediate hypersensitivity reactions, typically over
4 to 5 days.
746-750
Dave and Park
747
describe a protocol in which
heparin doses are continuously infused intravenously over 12-
hour periods in half-log 10 increments to a goal infusion of 1000
unit/h of heparin (Table LXXXIII). The patient successfully
tolerated a 50,000 unit bolus of heparin after completing this
desensitization and a subsequent smaller dose of heparin.
Management of HIT similarly involves immediate cessation of
the offending agent, use of alternative anticoagulants, and future
avoidance of both UFH and LMWHs.
739
Because of the nature of its use as a reversal agent, there is little
role for desensitization for intraoperative protamine. Manage-
ment for these reactions typically focuses on modification of
operative protocols where it is to be used, because there are no
clinically available alternatives to protamine. In the future, there
may be alternatives approved by the FDA; there are already
several products in advanced clinical phases.
751
Desensitization to protamine-containing insulin may be
necessary for diabetic patients who have no alternatives; this has
been described in the literature using the product neutral prot-
amine Hagedorn. One protocol successfully describes a starting
dose of 0.001 units intradermally, doubling every 20 to 30 mi-
nutes until a dose of 0.1 unit is reached. The protocol then
continues with subcutaneous injections until they reach the goal
dose of 4 units.
752
Coagulation factors (by Craig D. Platt, MD, PhD)
General.
The use of coagulation factors is the cornerstone of
treatment for hemophilia A (deficiency of factor VIII), hemo-
philia B (deficiency of factor IX), and type III von Willebrand
disease (vWD) (the most severe form of vWD).
753,754
TABLE LXXXI. Protocol for treating through cutaneous clopidogrel hypersensitivities
720
Initial therapy Continue clopidogrel 75 mg/d
Methylprednisolone taper (6-d Medrol Dosepak)
Antihistamines until symptom resolution (fexofenadine 180 mg/d and diphenhydramine 25-50 mg
at bedtime)
Secondary therapy for hypersensitivity reoccurrence Continue clopidogrel 75 mg/d
Longer course of corticosteroids (up to 18 d, eg, prednisone 60 mg, taper by 10 mg every 3 d)
Montelukast 10 mg/d
Antihistamines as needed
TABLE LXXXII. Anticoagulants by structural and functional category
Heparins Direct thrombin inhibitors Factor Xa inhibitors
UFH Hirudins Apixaban
LMWHs Bivalirudin Rivaroxaban
Ardeparin Desirudin
Certoparin Lepirudin
Dalteparin Argatroban
Enoxaparin Dabigatran etexilate
Nadroparin
Reviparin
Tinzaparin
Heparinoids
Danaparoid
Synthetic heparins
Fondaparinux
TABLE LXXXIII. Heparin desensitization protocol*
Step Solution (U/mL) Rate (U/h IV) Time (h) Volume infused per step (mL) Dose infused per step (units) Cumulative dose (units)
1 1 0.5 0-12 6 6 6
2 1 1.5 12-24 18 18 24
3 10 4.5 24-36 5.4 54 78
4 10 13.6 36-48 16.32 163.2 241.2
5 10 40.8 48-60 48.96 489.6 730.8
6 100 122.5 60-72 14.7 1,470 2,200.8
7 100 367.4 72-84 44.09 4,408.8 6,609.6
8 100 1008.0 84-96 120.96 12,096 18,705.6
The patient was preoperative for cardiac bypass and maintained at a dose of 1000 units/h until surgery. Additional desensitization protocols are reviewed in the same reference.
Available solution concentrations may vary depending on institution.
*Modified from Dave and Park.
747
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S83

Unfortunately, 2 major complications can prevent optimal
therapy in a subset of patients. First, inhibitory alloantibodies
(predominantly of the isotype IgG) to the exogenously supplied
coagulation factors can develop.
755,756
Such inhibitors substan-
tially increase the risk of morbidity and mortality due to
incomplete hemostasis. Second, acute hypersensitivity reactions,
often with features of anaphylaxis, can occur with in-
fusions.
755,756
In some cases, patients simultaneously develop
inhibitors and hypersensitivity reactions, a challenging clinical
scenario that significantly limits treatment options.
755,756
Major symptoms of hypersensitivity. Symptoms of
anaphylaxis associated with factor infusion have been most
commonly described in patients with hemophilia B. Although
only 3% to 5% of patients develop inhibitory antibodies, the
development of such antibodies is a major risk factor for
anaphylaxis.
755-757
Warrier et al
757
reviewed 18 such cases. The
clinical manifestations of hypersensitivity reactions were (listed
from most to least frequent) rash, bronchospasm, angioedema,
emesis, restlessness, cough, hypotension, and syncope.
757
Pa-
tients with complete factor IX gene deletion have been shown to
TABLE LXXXIV. Recommended immediate hypersensitivity testing for vWF-containing products and FVIII
762
Product SPT IDT 1 IDT 2
Wilate vWF 100 IU/mL, FVIII 100 IU/mL vWF 10 IU/mL, FVIII 10 IU/mL vWF 100 IU/mL, FVIII 100 IU/mL
Humate P vWF 120 IU/mL, FVIII 50 IU/mL vWF 12 IU/mL, FVIII 5 IU/mL vWF 120 IU/mL, FVIII 50 IU/mL
Helixate 300 IU/mL 30 IU/mL 300 IU/mL
Advate 300 IU/mL 30 IU/mL 300 IU/mL
Xytha 300 IU/mL 30 IU/mL 300 IU/mL
Monoclate 300 IU/mL 30 IU/mL 300 IU/mL
FVIII, Factor VIII; vWF, von Willebrand factor.
TABLE LXXXV. Recommended immediate hypersensitivity testing for factor IX
764
Product SPT IDT 1 IDT 2
Mononine 100 IU/mL 1 IU/mL 10 IU/mL
Monoclate 100 IU/mL 1 IU/mL 10 IU/mL
IU, International unit.
TABLE LXXXVI. Desensitization protocol to factor IX*
Day Dose (units/kg) Cumulative dose (units/kg) Method Interval from infusion of previous dose
Day 1 0.01 0.1 Slow IV push 0 min
0.02 0.3 Slow IV push 10 min
0.04 0.7 Slow IV push 10 min
0.08 0.15 Slow IV push 10 min
0.1 0.25 Slow IV push 10 min
0.2 0.45 Slow IV push 20 min
0.4 0.85 Slow IV push 20 min
0.8 1.65 Slow IV push 20 min
1.5 3.15 Slow IV push 20 min
3.0 6.15 Continuous infusion over 30 min —
6.0 12.15 Continuous infusion over 30 min —
8.0 20.15 Continuous infusion over 30 min —
9.0 29.15 Continuous infusion over 60 min —
11.0 40.15 Continuous infusion over 60 min —
12.0 52.15 Continuous infusion over 60 min —
14.0 66.15 Continuous infusion over 60 min —
16.0 82.15 Continuous infusion over 60 min —
18.0 100.15 Continuous infusion over 60 min —
Day 2 100 100 Continuous infusion over 10 h —
Day 3 100 100 Continuous infusion over 8 h —
Day 4 100 100 Continuous infusion over 6 h —
Day 5 100 100 Continuous infusion over 4 h —
Day 6 100 100 Continuous infusion over 2 h —
Day 7 100 100 Continuous infusion over 1 h —
Day 8 100 100 Continuous infusion over 30 min —
*This protocol was devised and used successfully, given severe persistent urticaria with a standard 12-step approach.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S84 BROYLES ET AL

be at the greatest risk of developing inhibitors and anaphy-
laxis.
757
There does not seem to be a single common mechanism
for anaphylaxis, because the development of IgG
1
, IgG
4
, and IgE
to factor IX has been described.
756
Although up to 30% of patients with hemophilia A receiving
factor VIII replacement develop inhibitors, anaphylaxis is a very
rare complication.
756
Unlike in hemophilia B, there does not
appear to be a correlation with anaphylaxis and the development
of inhibitory antibodies.
756
Published reports of such reactions
have included respiratory distress, hypotension, and diffuse ur-
ticaria or erythroderma.
758,759
In several cases, allergic reactions
to factor VIII infusions have been presumed to be triggered by
concentrate components other than the factor VIII itself.
759,760
Inhibitory antibodies develop in approximately 10% of pa-
tients with type III vWD.
761,762
Anaphylaxis has been reported
in a number of these patients, though good estimates of the
incidence of anaphylaxis in patients with inhibitors is lack-
ing.
719,756,761-763
Two siblings with type III vWD have been
recently described, one of whom developed shortness of breath,
urticaria, tachycardia, and mild hypotension. His sibling’s
symptoms were limited to respiratory distress and back pain.
762
Diagnosis. Skin testing protocols have been described for
factor XIII (Table LXXXIV), factor IX (Table LXXXV), and von
Willebrand factor (Table LXXXIV).
762,764,765
However, in most
cases, IgG alloantibodies are responsible for the reactions and
these antibodies are not detected on such testing.
755,756
Patients
with hemophilia B and vWD with documented inhibitory an-
tibodies should be considered at risk for anaphylaxis with factor
infusions.
755,756
Patients with hemophilia A, even those with
high levels of inhibitors, are not considered at elevated risk of an
acute hypersensitivity reaction.
755,756
Management. The use of bypassing agents recombinant factor
VII activated and activated prothrombin complex concentrates
including factor VIII inhibitor bypassing activity has been
described for acute bleeding episodes in patients with hemophilia
A, hemophilia B, and vWD.
766,767
Desensitization protocols have
been published for all 3 disorders as well (Tables LXXXVI and
LXXXVII).
762-765
For patients with inhibitors to these factors,
such protocols can be the first step of an immune tolerance in-
duction protocol.
762,768
It is important to note that nephrotic
syndrome is a relatively common complication of immune toler-
ance induction in patients with hemophilia B and therefore
episodic control with recombinant factor VII activated rather than
immune tolerance induction should be strongly considered.
768
OTHER DRUGS
Radiographic contrast media (by Knut Brockow,
MD)
General.
Adverse reactions to iodinated radiocontrast media
(RCM) may be either chemotoxic (eg, cardiotoxicity, neurotoxicity,
and nephrotoxicity) or due to hypersensitivity.
769
Hypersensitiv ity
reactions manifest either immediately (<1 hour of administration)
or are nonimmediate responses (>1 hour). Immediate hypersensi-
tivity reactions present with anaphylaxis, and nonimmediate re-
actions predominantly are exanthems.
769
The pathogenesis is
normally related to the molecular RCM structure and not to iodine
or to seafood allergy.
770
Thereisincreasingevidencethatsomeof
these reactions may be immunologic and that allergy tests may help
to identify agents that may be tolerated.
769,771
Mild immediate
TABLE LXXXVII. Twelve-step desensitization protocol to wilate*
Full therapeutic dose: 435 IU daily
Premedication Diphenhydramine†
A. Prepared solutions
Solution mL/bag IU/bag IU/mL
1 250 4.35 0.017
2 250 43.5 0.174
3 250 431.6 1.726
B. Desensitization
Step Solution Rate (mL/h) Time (min) Dose (IU) Cumulative dose (IU)
1 1 2 15 0.009 0.009
2 1 5 15 0.022 0.031
3 1 10 15 0.044 0.074
4 1 20 15 0.087 0.161
5 2 5 15 0.218 0.379
6 2 10 15 0.435 0.814
7 2 20 15 0.870 1.684
8 2 40 15 1.740 3.424
9 3 10 15 4.316 7.739
10 3 20 15 8.632 16.371
11 3 40 15 17.263 33.634
12 3 75 186 401.37 435.000
Total time 351 min
IU, International unit.
*The total IU dose injected is more than the final dose because solutions 1 and 2 are not completely infused.
†Oral dose of 1 mg/kg, 1 h before start of desensitization and 1 h before each subsequent dose.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S85

hypersensitivity reactions, such as urticaria and pruritus, have been
reported to occur in 0.7% to 3.1% of patients receiving modern
nonionic RCM, whereas severe life-threatening reactions occur in
0.02% to 0.04% of patients, and fatal hypersensitivity in 1 to 3 per
100,000 RCM administrations.
769
The fre quency of reported
nonimmediate exanthems varies greatly and has been reported to
affect about 1% to 3% of RCM-exposed patients.
769
Major symptoms. Immediate RCM hypersensitivity re-
actions manifest with symptoms of anaphylaxis.
771
Pruritus and
urticaria with or without angioedema are most common.
Gastrointestinal symptoms such as nausea, vomiting, abdominal
pain, and diarrhea may occur. Reactions may involve the respi-
ratory and cardiovascular systems and present with dyspnea,
bronchospasm, and/or a sudden drop in blood pressure. Hypo-
tension may be associated with loss of consciousness (anaphy-
lactic shock). The onset of immediate hypersensitivity reactions
is within 5 minutes after injection in about 70% of reactions
771
;
96% of severe or fatal reactions manifest within 20 minutes.
Nonimmediate RCM hypersensitivity reactions are usually
mild to moderate in severity and are self-limiting. The typical
clinical manifestation is a maculopapular exanthem occurring
within a few hours to several days after the RCM administra-
tion.
771
Uncommon skin reactions including erythema, urticaria,
angioedema, fixed drug eruption, erythema multiforme, SJS,
TEN, and papulopustular eruptions have been described.
771
Diagnosis. For immediate hypersensitivity reactions, anaphy-
laxis may be confirmed by obtaining blood samples for histamine
analysis drawn immediately, or for tryptase ideally drawn 1 to 2
hours after the onset of symptoms. The further allergy workup
should be performed within 6 months after the reaction for best
test results (Figure 8).
771
It consists of a SPT with undiluted RCM
followed by IDT with RCM (300-320 mg/mL) diluted 10-fold in
sterile saline and reading after 20 minutes (Table LXXXVIII).
771
In case of a positive reaction, a panel of several different RCM
should be tested. There is no commercial assay for RCM-specific
IgE antibodies. Increased basophil activation to RCM has been
described, but the reliability of this or other in vitro tests has not
yet been sufficiently established. Several studies from Europe and
Asia have shown that skin testing may be helpful in patients with
past hypersensitivity reactions to RCM. Skin tests with RCM will
give positive results only in a minority (w10%-25%) of imme-
diate and 30% to 70% of nonimmediate reactions with clinical
pictures typical for drug hypersensitivity reactions.
771,772
For nonimmediate exanthems, it is recommended to use SPT
and patch tests with undiluted RCM and IDTs with 10-fold
diluted products in physiologic saline with delayed readings after
48 and 72 hours (in case of local pruritus or erythematous pla-
ques also at additional time points; Table LXXXVIII).
771
RCM-
related T-cell activity may additionally be assessed in vitro by
lymphocyte transformation test and by lymphocyte activation
test, although their sensitivity and specificity remain unknown,
and these tests are not commercially available.
Drug challenge has not been generally recommended because
intravenous applications of as low as 1 mL RCM have anec-
dotally led to severe anaphylaxis. However, evidence is increasing
that, in experienced centers, challenge tests can be performed to
confirm results of skin tests.
773
Graded drug challenge tests have been recommended to confirm
negative skin test results, for example, 1/10 of the full dose on day 1,
1
/
2
of the full dose on day 2, and the full dose on day 3 at the
radiology department, becau se a neg ative skin test result does
indicate, but does not necessarily guarantee, tolerance.
769
At this
time there is inadequate published experience in the pediatric
population; therefore, the approach to hypersensitivity to RCM in
children has been modeled from the one used for adults.
Management. Patients with previous hypersensitivity reactions
to RCM are at risk for developing new reactions on reexposure.
769
If such patients need another contrasted examination, the culprit
preparation should be avoided, particularly if there is a history of
severe reactions. In those with positive skin test reaction to the
culprit, cross-reactivity to other RCMs is common.
772
The risk of
RCM cross-reactivity to different RCMs appears to be related to
the structure of the culprit. Three different groups of RCM have
been proposed, with frequent reactions within groups and scarce
skin test positivity among RCMs of different groups.
772
In case of
a positive reaction, a skin testenegative product should be iden-
tified. The preventive value for the selection of an alternative
RCM by skin test still has to be further confirmed. The benefitis
limited to those patients with positive skin tests to RCM. In the
United States, skin testing after immediate reactions to RCM is
not considered standard of care; however, the role of skin tests in
the evaluation is evolving. In 1 study, however, the negative pre-
dictive value of reapplication of RCM in combination with skin
tests was reported to be high.
773
A fractionated challenge test may
be considered in nonimmediate exanthems. In patients with
negative skin test result or in whom skin tests are not performed,
the use of premedication with antihistamines and/or glucocorti-
coids is no longer recommended. The anaphylaxis 2020 practice
–
negative negative
+
–
+
positive
Adverse reaction to RCM
Chemotoxic,
unspecific or
unrelated event
No test
Typical symptoms of
allergic reactions
Urticaria, symptoms of
anaphylaxis (immediate)
Exanthema
(nonimmediate)
SPT followed by IDT,
reading after 20 minutes
(optional: BAT)
SPT, IDT, PT, reading after
20 minutes, 48-72 hours
(optional: LTT, LAT)
Skin test
alternative agents
Skin test
alternative agents
Consider doing
provocation test
Consider doing
provocation test
Check necessity, use test-negative
alternative, after severe reactions
consider additional premedication
1. Use other nonrelated RCM
2. Consider premedication (eg, 50 mg prednisone 13, 7,
and 1 hour and 50 mg diphenhydramine 1 hour
before the procedure for immediate reactions and
50 mg prednisone 13, 7, and 1 hour before the
procedure
for nonimmediate exanthemas)
3. Emergency preparedness
FIGURE 8. Test procedure in RCM hypersensitivity.
769
LAT,
lymphocyte activation test; LTT, lymphocyte transformation test.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S86 BROYLES ET AL

parameter update performed a systematic review and Grading of
Recommendations, Assessment, Development, and Evaluation
(GRADE) analysis and suggested against premedication to prevent
anaphylaxis in patients with prior radiocontrast HSRs when
readministration of a low- or iso-osmolar, nonionic RCM agent is
required.
774
This was a conditional recommendation with the
certainty rating of evidence as being very low. Therefore pre-
medication may be considered in clinical circumstances associated
with a high level of perceived risk of anaphylaxis or comorbidities
associated with greater anaphylaxis fatality risk (such as underlying
cardiovascular disease, use of beta-blockers, or prior severe
anaphylaxis), although evidence is lacking to clearly support this
practice and breakthrough reactions may still occur. Corticoste-
roids (eg, 50 mg prednisone 13, 7, and 1 hour before the pro-
cedure) and H
1
(eg, 50 mg diphenhydramine 1 hour before the
procedure) H
2
antihistamines are the most frequently recom-
mended premedication agents. Physicians dealing with these pa-
tients should not rely on the efficacy of premedication.
Corticosteroids (by Iris Otani, MD, and Rima Rachid,
MD)
Background.
Corticosteroids are used in the treatment of
several con ditions, i ncluding al lergi c conditi ons, malignancy,
autoimmunity, and transplantation. Immediate
TABLE LXXXVIII. Skin test concentrations for radiocontrast media*
Test Concentration*
Readings
Immediate reaction Nonimmediate reaction
Skin prick test Undiluted 20 min 20 min, 48 h, 72 h†
Intradermal test 1/10 diluted 20 min 20 min, 48 h, 72 h†
Patch test Undiluted 20 min, 48 h, 72 h†
*Radiocontrast media with an iodine concentration of 300-320 mg/mL.
†If the patient notices a positive reaction (pruritus, erythema) at the skin test site at other time points, additional readings may be performed (eg, after 24 h or 96 h).
TABLE LXXXIX. Recommended immediate hypersensitivity testing for corticosteroids and excipients*
777-781,792,802,804
Corticosteroid SPT dilutions (mg/mL) IDT dilutions (mg/mL) References
Betamethasone sodium phosphate 4-6 0.006 SPT: Asakawa, Figueredo, Venturini, Rachid
0.06 IDT: Figueredo, Rachid
0.6
4-6
Betamethasone acetate 6 6 SPT: Mace
IDT: Montoro
Budesonide 0.25 0.0025 SPT: Venturini
0.025 IDT: Venturini
Dexamethasone sodium phosphate 4 0.004 SPT: Mace, Venturini, Rachid
0.04 IDT: Rachid, Baker, Venturini
0.4
4
Hydrocortisone sodium succinate 10-100 0.01-1 SPT: Figueredo, Venturini, Rachid
0.1-10 IDT: Rachid, Venturini, Montoro
10-25
Methylprednisolone acetate 40 0.4 ST: Mace, Venturini
4 IDT: Baker, Venturini
Methylprednisolone sodium succinate 10-40 0.01 ST: Mace, Rachid
0.1-0.4 IDT: Baker, Rachid
4-10
Prednisone 3-30 No IDT Venturini, Rachid
Prednisolone 3-10 No IDT Venturini, Rachid
Triamcinolone acetonide 10-40 0.01 SPT: Venturini, Rachid
0.1-0.4 IDT: Baker, Venturini, Montoro, Rachid
1-4
10-40
Polyethylene glycol† 10 (1:100) 0.1 (1:10,000) SPT: Sohy
100 (1:10) 1 (1:1,000)
10 (1:100)
Carboxymethylcellulose† 5 0.005 Venturini
0.05
*Dilutions adapted from cited publications.
†Dilutions based on literature and not personal experience.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S87

hypersensitivity reactions to corticosteroids are overall rare.
The exact incidence is unknown, but hypersensitivity reactions
to corticosteroids have been reported with an estimated pre v-
alence of 0.1% to 0.3%.
Clinical presentation. Most immediate h ypersensitivity
reactions to corticosteroids occur within an hour of adminis-
tration. Symptoms may include 1 or more of the following:
urticaria, angioede ma, wheezing, bronchospasm, nausea,
vomiting, hypotension, or even cardiovascular collapse.
775,776
Recognition of corticosteroids as the culprit for these reactions
is sometimes challenging, because they are often used to treat
conditions that may lead to similar symptoms, such as allergic
reactions, status asthmaticus, anaphylaxis, or shock. Hence,
hypersensitivity reactions to corticosteroids may go unrecog-
nized and patients may develop more than 1 reaction before
thisdiagnosisissuspected.Itisnotclearwhethersometypesof
corticosteroids are more prone to causing hypersensitivity re-
actions than others, or whether the likelihood of reactions is
related to the frequency of administration of specificcortico-
steroids. In a recent review of the literature from 2004 to 2014,
120 hypersensitivity reactions to corticosteroids were reported
in 106 patients. Methylprednisolone was impl icat ed in 41% of
all types of reactions, followed by prednisolone (20%),
triamcinolone (14%), and hydrocortisone (10%).
775,776
Re-
actions occur most commonly after intravenous (44% of cases)
and oral (26%) administration, though reports of reactions
after intra-articular, ophthalmic, and topical administration
have also been published.
775-777
Diagnosis and management of imm ediate hyper-
sensitivity reactions.
Identification of an alternative cortico-
steroid for future use is possible in most cases with skin prick and
intradermal testing at nonirritating concentrations (Table LXXXIX)
andgradedchallenge.
775,776 ,778-781
Whenever possible, testing
should be performed with a preservative-free corticosteroid, in
addition to preservative testing if needed. Negative skin test results
should be confirmed with drug challenge.
782-800
Cross-reactivity
patterns based on structural characteristics have not been clearly
established for immediate hypersensitivity reactions as they have
been for delayed reactions as described by Coopman et al.
801-804
Some reports suggested that hydrocortisone is more cross-reactive
with methylprednisolone than with halogenated corticosteroids such
as dexamethasone and betamethasone, whereas others did not find a
defin ite pattern of cross-reactivity based on the history and skin and
intradermal testing.
802,805,806
Hydrophobic corticosteroids are sometimes chemically
bonded to an ester such as sodium succinate or sodium phos-
phate to transform them into soluble injectable products. Im-
mediate hypersensitivity reactions have been reported to the
succinate ester component of the corticosteroids.
784,786,804,807-
811
Hence, when evaluating a reaction that is thought to be
secondary to esterified corticosteroids, it is recommended to
include the suspected corticosteroids in the skin and intradermal
testing, and, in addition, the same corticosteroids without the
ester component or with a different ester.
Immediate hypersensitivity reactions can also occur because of
excipients or preservatives in a corticosteroid preparation. Sen-
sitivities to lactose, carboxymethylcellulose, polyethylene glycol,
and hexylene glycol have been reported.
782,783,785,787,789-
793,803,812-816
When preservatives or excipients are present in the
corticosteroid preparation, skin testing with these agents can be
considered. Skin testing with milk proteins can be considered in
patients with milk allergy reacting to lactose-containing corti-
costeroid preparations.
Desensitization to methylprednisolone has been successfully
performed and is an option when an alternative therapeutic agent
cannot be identified (Table XC).
807
Contact dermatitis (delayed hypersensitivity) to
topical corticosteroids
Background.
Contact dermatitis to topical corticosteroids has
been reported with a frequency of 0.5% to 5%.
788
Contact
dermatitis to corticosteroids occurs more frequently in women
(3:1 women to men), and among the following occupations:
housewife (18%), office work (17%), retired (7%), housekeeping
(6%), education (5%), student (5%), and health care (4%).
795
Diagnosis and management. Patch testing is the stan-
dard fo r diagnosing con tact dermatitis.
797
A combination of
TABLE XC. Previously published desensitization protocol for methylprednisolone
807
Bag Volume per bag (mL) Concentration (mg/mL)
1 250 0.040
2 250 0.400
3 250 3.969
Step Bag Rate (mL/h) Time (min) Administered volume (mL) Administered dose (mg) Cumulative dose (mg)
1 1 2 15 0.5 0.02 0.02
2 1 5 15 1.25 0.05 0.07
3 1 10 15 2.5 0.1 0.17
4 1 20 15 5 0.2 0.37
5 2 5 15 1.25 0.5 0.87
6 2 10 15 2.5 1 1.87
7 2 20 15 5 2 3.87
8 2 40 15 10 4 7.87
9 3 10 15 2.5 9.921 17.791
10 3 20 15 5 19.843 37.634
11 3 40 15 10 39.685 77.319
12 3 80 174.375 232.5 922.681 1000
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S88 BROYLES ET AL

tixocortol-21-pivalate and budesonide included in the thin-
layer r apid use epic utaneous test can id entif y 9 1.3% of
corticosteroid-sensitivity patients.
796
Supplemental patch
testing is needed to identify the culprit corticosteroid in the
remaining patients. Baeck et al
795
provide comprehensive
vehicle and c oncen tration recommendati ons fo r corti coster oid
patch testing in their review. Cross-reactivity patterns within
modified Coopman classification groups have been observed
for delayed reactions (Table XCI).Forpatientswithpositive
reactions to tixocortol-21-pivalate or budesonide on thin-layer
rapid use epicutaneous test, consideration of cross-reactivity
patterns and supplemental patch testing can help identify
corticosteroids that can be tolerated for future therapeutic
use.
Vehicles used for topical corticosteroid preparations can also
cause irritation or contact dermatitis. Parabens, formaldehyde-
releasing preservatives (quaternium-15), isothiazolinones, lanolin,
ethylenediamine, sorbitan sesquioleate, fragrance, and propylene
glycol are all known contact allergens commonly used in topical
corticosteroid preparations.
794,798-800,817-819
The thin-layer rapid
use epicutaneous test includes all these additives except for pro-
pylene glycol, for which testing can be performed with 30%
aqueous solutions, and sorbitan sesquioleate, for which testing can
be performed with 20% petrolatum preparations.
820,821
Proton pump inhibitors (by Anna Wolfson, MD)
General.
Proton pump inhibitors (PPIs) are widely used in-
hibitors of gastric acid secretion. PPIs are generally well tolerated,
with a 1% to 2% risk of minor adverse reactions with rare reports
of serious adverse events.
822
Hypersensitivity is also rarely
described.
823-825
PPIs are modified benzimidazoles that contain a pyridine ring
and unique side-chain substitutions. Omeprazole and pan-
toprazole have a methoxy and a difluoromethoxy chain in their
benzimidazole rings, respectively, whereas lansoprazole and
rabeprazole have a trifluoroethoxy and methoxypropoxy chain in
their pyridine rings, respectively. Multiple case reports have
described possible cross-reactivity among PPIs, based on the
underlying structural similarity and confirmed by skin
testing.
823,826-828
Three patterns of cross-reactivity are described
on the basis of combining the data from the most recent
literature.
829
1. Hypersensitivity to omeprazole showed cross-reactivity with
all other PPIs.
825,828
2. Hypersensitivity to omeprazole showed cross-reactivity with
pantoprazole, or with pantoprazole and esomeprazole or
rabeprazole, but not with lansoprazole.
824-826,828
3. Hypersensitivity to lansoprazole showed cross-reactivity with
1 (omeprazole, pantoprazole, rabeprazole, esomeprazole) but
not with any of the other PPIs.
824,828,830,831
Major symptoms of hypersensitivity. Among the PPIs,
lansoprazole, followed by omeprazole, is most frequently impli-
cated as the cause of immediate hypersensitivity.
823,824
IgE-
mediated reactions are most commonly reported, accounting for
86% of all hypersensitivity reactions in a recent review of 118
cases.
823
In this same review, the clinical manifestations of hy-
persensitivity reactions were (listed from most to least frequent)
urticaria, generalized itching or pruritus, angioedema, hypoten-
sion, skin rash other than urticaria, erythema, and dyspnea or
shortness of breath.
823
The clinician should keep in mind that,
TABLE XCI. Observed cross-reactivity patterns within corticosteroid groupings based on the classification by Coopman et al
801
and
modified by Matura and Goossens
788
Group Structure Cross-reactivity
A—Hydrocortisone type
Hydrocortisone
Methylprednisolone
Prednisolone
Short-chain ester or thioester on C
21
Within group
With budesonide-(S)-isomer and group D2
corticosteroids
B—Triamcinolone acetonide type
Desonide
Fluocinolone
Tramcinolone
C
16
,C
17
-cis-ketal or -diol Within group
Budesonide-(S)-isomer cross-reacts with
group A and D2 corticosteroids
C—Betamethasone type C
16
-methyl substitution
C1
Betamethasone
Dexamethasone
Desoximetasone
Nonesterified Betamethasone and/or dexamethasone and
group B
C2
Diflucortolone
Fluocortolone
Clocortolone
Stable esters (-valerate, -propionate, -diflucortolone
valerate, -flumethasone pivalate)
No significant cross-reactivity pattern
observed
D—Hydrocortisone-17-butyrate type Long-chain ester at C
17
or C
17
and C
21
with or without
C
16
-methyl substitution
D1
Clobetasol-17-propionate
Betamethasone dipropionate
Mometasone furoate
Aclometasone dipropionate
C
16
methyl substitution on B ring Rare cross-reactivity between aclomethasone
dipropionate and group A, budesonide,
group D2
D2
Hydrocortisone-17-butyrate
Hydrocortisone valerate
Lacks long-chain ester and methyl substitution
Lipophilic prodrugs that penetrate skin easily
Within group
With group A corticosteroids
With budesonide-(S)-isomer
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S89

because many of the drugs are delayed release preparations, pa-
tients may present with hypersensitivity symptoms 2 to 3 hours
after ingestion as opposed to the classic teaching of 1 hour.
823
The remaining 14% of cases were noneIgE-mediated and
included 1 case of DRESS to esomeprazole (a subsequent case
reported DRESS to omeprazole
832
), 1 case of TEN to lanso-
prazole, 1 case of hypersensitivity vasculitis, and 5 cases of
contact dermatitis (3 of which were occupational exposures, 1 to
lansoprazole and 2 to omeprazole).
823
A subsequent large case
series of 96 cases of occupational sensitization to omeprazole
identified 36 patients with evidence of sensitization to omepra-
zole based on patch testing and lymphocyte transformation
test.
833
Acute interstitial nephritis has been well described with
PPI use.
834
Subacute cutaneous lupus erythematosus has also
been described.
835
Diagnosis. The validity of skin testing for the evaluation of
immediate hypersensitivity to PPIs has been studied in a pro-
spective analysis by Kepil Ozdemir et al,
824
who reported a
sensitivity of 58.8%, specificity of 100%, negative predictive
value of 70.8%, and positive predictive value of 100%. Their
skin testing protocol is outlined in Table XCII as well as the
protocol proposed by Bose et al,
823
which is based on a broader
literature review. In clinical experience, cross-reactivity among all
the PPIs can be seen.
836
Unfortunately, the low negative pre-
dictive value of skin testing means that this is often observed
during the oral challenge. The BAT, when used in conjunction
with skin testing, has been described as being a useful guide for
whether oral challenge should be performed.
837
Patch testing has been used in cases of suspected delayed
hypersensitivity. Ghatan et al
833
used an omeprazole sodium salt
TABLE XCII. Recommended immediate hypersensitivity testing for PPIs
823,824
PPI
Bose et al
823
Kepil Ozdemir et al
824
SPT dilutions (mg/mL) IDT dilutions (mg/mL)* SPT dilutions (mg/mL) IDT dilutions (mg/mL)*
Omeprazole 40 0.04 0.4 0.004
0.4 4 0.04
4 20 0.4
Esomeprazole 40 0.04 0.8 0.008
0.4 8 0.08
4 20 0.8
Lansoprazole 30 0.03 30 None
0.3
3
Pantoprazole 40 0.04 0.4 0.004
0.4 4 0.04
4 40 0.4
Rabeprazole 20 0.02 20 None
0.2
2
*Intradermal testing should only be performed using injectable intravenous preparations of PPIs.
TABLE XCIII. Oral desensitization protocol for omeprazole*
839
Time (h) Concentration (mg/mL) Dose (mL) Dose (mg) Cumulative dose (mg)
0:20 0.002 0.5 0.001 0.001
0:40 0.002 1 0.002 0.003
1:00 0.002 2 0.004 0.007
1:20 0.002 4 0.008 0.015
1:40 0.02 0.5 0.01 0.025
2:00 0.02 1 0.02 0.045
2:20 0.02 2 0.04 0.085
2:40 0.02 4 0.08 0.165
3:00 0.2 0.5 0.1 0.265
3:20 0.2 1 0.2 0.465
3:40 0.2 2 0.4 0.865
4:00 0.2 4 0.8 1.665
4:20 2 0.5 1 2.665
4:40 2 1 2 4.665
5:00 2 2 4 8.665
5:20 2 4 8 16.665
5:40 2 8 16 32.665
*Omeprazole granules were dissolved into bicarbonate solution to create serial dilutions.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S90 BROYLES ET AL

in saline solution at 0.1%, 0.5%, and 1% for patch testing. In
the case of severe, life-threatening delayed reactions to PPIs, such
as DRESS and TEN, reexposure to a suspected culprit for testing
purposes must be performed with extreme caution and empiric
avoidance is recommended.
Management. Many patients with hypersensitivity to a PPI
can tolerate an alternative PPI, and skin testing (usually to the
culprit PPI, and to at least 1 other PPI) followed by oral chal-
lenge can identify candidate alternates.
838
Finally, a successful
desensitization protocol to omeprazole in the context of imme-
diate IgE-mediated hypersensitivity has been published
(Table XCIII).
839
Allopurinol (by Alberta L. Wang, MD)
General.
Allopurinol is the primary therapeutic agent used for
the treatment of gout and hyperuricemia. Most allopurinol hy-
persensitivity reactions are delayed, with a median time of onset
of 3 weeks, but reactions have been reported to occur years after
initiation of therapy.
840
Rash is the most common manifestation
of allopurinol hypersensitivity, with an overall incidence of
approximately 2%.
841
SCARs to allopurinol, including DRESS,
SJS, and TEN, are rare but have a high associated mortality of up
to 27%.
842
Allopurinol is rapidly metabolized by xanthine oxidase to
oxypurinol, which has a half-life of 23 hours, and is excreted by
the kidney. Allopurinol hypersensitivity is primarily mediated by
CD4
þ
and CD8
þ
T-cell responses to oxypurinol.
843
The risk of
hypersensitivity reaction is increased by factors that decrease
oxypurinol excretion, including impaired kidney function and
concurrent diuretic use. The HLA-B*58:01 allele is also associ-
ated with an increased risk for allopurinol hypersensitivity.
844
There is an increased prevalence of allopurinol hypersensitivity in
Asian subpopulations, which has been correlated with higher
HLA-B*58:01 allele frequencies. HLA-B*58:01 screening is
recommended for patients of Korean ethnicity with chronic
kidney disease stage 3 or worse and patients of Han Chinese or
Thai ethnicity irrespective of renal function before initiation of
allopurinol.
845
Major symptoms of hypersensitivity. The most com-
mon hypersensitivity reaction to allopurinol is a maculopapular
exanthem. The incidence of SCARs, including DRESS, SJS, and
TEN, is rare (w0.1%). However, allopurinol users have a 10
times higher relative risk of a SCAR compared with nonusers,
and allopurinol is a common cause of SJS and TEN.
846,847
In
addition, systemic hypersensitivity with acute interstitial
nephritis has been described in case reports.
848,849
TABLE XCIV. Rapid 2-d oral desensitization protocol for immediate hypersensitivity to allopurinol
Interval (min) Solution* Volume (mL) Dose (mg)
Day 1 B 0.25 0.05
30 B 0.5 0.1
30 B 1 0.2
30 B 2.5 0.5
30 B 5 1
30 A 2.5 5
30 A 5 10
30 A 12.5 25
30 Half 100-mg tablet — 50
30 100-mg tablet — 100
Day 2 100-mg tablet — 100
60 Two 100-mg tablets — 200
*Solution A: Crush 2 tablets of 100 mg allopurinol into a volume of 100 mL diluent (Ora-Plus/Ora-Sweet 1:1) to a fi nal concentration of 2 mg/mL; Solution B: 1:10 dilution of
solution A to a final concentration 0.2 mg/mL.
TABLE XCV. Twenty-eight-day allopurinol desensitization protocol for delayed hypersensitivity*
Days Daily dose Allopurinol administration instructions (by mouth)
1-3 50
m
g 0.25 mL of suspension A
4-6 100
m
g 0.5 mL of suspension A
7-9 200
m
g 1 mL of suspension A
10-12 500
m
g 2.5 mL (
1
/
2
tsp) of suspension A
Then change to suspension B
13-15 1 mg 1 mL of suspension B
16-18 5 mg 5 mL (1 tsp) of suspension B
19-21 10 mg 10 mL (2 tsp) of suspension B
22-24 25 mg
1
/
4
of a 100-mg tablet or 25 mL (5 tsp) of suspension B
25-27 50 mg
1
/
2
of a 100-mg tablet
28 and on 100 mg 1 full 100-mg tablet
tsp, Teaspoon.
*Suspension A: Allopurinol 0.2 mg/1 mL (100 mL); Suspension B: allopurinol 1 mg/1 mL (200 mL).
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S91

Diagnosis. Diagnosis is based on clinical history and symptom
recognition, in particular, the temporal relationship between
allopurinol initiation and symptom onset. Allopurinol skin
testing concentrations for IgE-mediated reactions are not stan-
dardized or validated. Intradermal skin testing with 0.1
m
gof
allopurinol has been reported with negative result in a patient
who presented with IgE-mediated symptoms.
850
Patch testing
with allopurinol has also been reported with low sensitivity.
842
Drug patch testing for systemic hypersensitivity reactions is not
standardized and the predictive value is unknown.
13
Management. The mainstay of treatment is cessation of
allopurinol and supportive care. For patients with a continued
indication for urate-lowering therapy, an alternative agent should
be considered. If allopurinol treatment is necessary and the initial
hypersensitivity reaction was not severe, desensitization can be
performed on an individual basis weighing the risks and benefits
of treatment. The type and severity of the initial reaction, patient
comorbidity, and urgency of treatment should be considered
when choosing a desensitization protocol.
In patients with a history of IgE-mediated reaction to allo-
purinol, case reports have described success with rapid intrave-
nous desensitization.
851
In addition, a rapid 2-day oral
desensitization protocol with a target dose of 200 mg has been
published (Table XCIV).
852
Published desensitization protocols for DHRs range from 16
to 78 days.
13,853,854
The standard 28-day oral desensitization
protocol (Table XCV) is generally well tolerated.
853
The 28-day
protocol balances the reduction of breakthrough reactions during
desensitization, such as fever and rash, and the length of
desensitization. The 28-day protocol reaches a target dose of 100
mg/d. For a target dose higher than this, one can continue to
increase the dose up to 2-fold every 3 days as tolerated. The
protocol can be modified with dosage adjustments and by
lengthening the time between steps if breakthrough reactions
occur. In patients who need allopurinol more urgently, a shorter
16-day oral desensitization protocol that reaches a target dose of
300 mg can be used (Table XCVI).
853
A longer 78-day oral
desensitization protocol is recommended for high-risk patients
who are frail or elderly with multiple medical comorbidities,
TABLE XCVI. Sixteen-day allopurinol desensitization protocol for delayed hypersensitivity*
Day Solution Amount Dose (mg)
1 A 1 mL 0.3
2 A 2 mL 0.6
3 A 4 mL 1.2
4 A 8 mL 2.4
5 A 10 mL 3
6B 3mL 18
7B 6mL 36
8 B 10 mL 60
9 100-mg tablet
3
/
4
tablet 75
10 100-mg tablet 1 tablet 100
11 100-mg tablet 1
1
/
4
tablet 125
12 100-mg tablet 1
1
/
2
tablet 150
13 100-mg tablet 1
3
/
4
tablet 175
14 100-mg tablet 2
1
/
4
tablet 225
15 100-mg tablet 2
1
/
2
tablet 250
16 300-mg tablet 1 tablet 300
*Source solution: 150-mg allopurinol tablet in 50 mL 5% dextrose (final concentration 3 mg/mL); Solution A: 1:10 source solution (0.3 mg/mL); Solution B: 300-mg allopurinol
tablet in 50 mL 5% dextrose (final concentration 6 mg/mL).
TABLE XCVII. Seventy-eight-day allopurinol desensitization protocol for delayed hypersensitivity
Daily dose Concentration/tablet Amount Days
10
m
g 1 mg/5 mL 0.05 mL 1-7
25
m
g 1 mg/5 mL 0.12 mL 8-14
50
m
g 1 mg/5 mL 0.25 mL 5-21
100
m
g 1 mg/5 mL 0.5 mL 22-28
200
m
g 1 mg/5 mL 1 mL 29-35
500
m
g 1 mg/5 mL 2.5 mL 36-42
1 mg 1 mg/5 mL 5 mL 43-49
5 mg 10 mg/5 mL 2.5 mL 50-56
10 mg 10 mg/5 mL 5 mL 57-63
25 mg 10 mg/5 mL 12.5 mL 64-70
50 mg 100-mg tablet
1
/
2
tablet 71-77
100 mg 100-mg tablet 1 tablet 78
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S92 BROYLES ET AL

renal impairment, or history of widespread cutaneous eruptions
with fever (Table XCVII).
13
Although allopurinol desensitization
is largely successful, there have been reports of unmanageable
recurrent rash and significant adverse reactions necessitating
discontinuation of treatment.
13,854
Therefore, continued vigi-
lance and monitoring are essential.
Antiepileptics (by Sarah L. Garon, MD, and Elizabe th
Phillips, MD)
General.
Aromatic antiepileptic drugs such as carbamazepine,
oxcarbazepine, phenytoin, phenobarbital, lamotrigine, felbamate,
and zonisamide have been associated with a number of
hypersensitivity drug reactions, and a clinical presentation of
benign, delayed rash is the most common manifestation (rates
ranging from 5% to 17%). On the spectrum of potentially severe
immunologically mediated adverse drug reactions (IM-ADRs),
isolated benign exanthem is most common and mild; however,
more SCARs exist, which include DRESS, AGEP, fixed drug
eruption, and SJS/TEN.
855
These SCAR syndromes differ ac-
cording to their clinical presentation, morbidity and mortality,
time between initiation of the drug and onset of symptoms,
immunopathogenesis, HLA and pharmacogenomic associations,
and utility of in vivo (patch and delayed prick/intradermal)
testing (Table XCVIII).
353,856,857
SCARs are not rare in
TABLE XCVIII. Pharmacogenomics of severe immunologically mediated adverse reactions associated with antiepileptic drugs
353,868
Drug Clinical phenotype Genetic association Population
Carbamazepine SJS/TEN HLA-B*15:02
HLA-B*15:21*
Han Chinese, Thai, Malaysian, Hindu Indian
HLA-B*15:11 Korean, Japanese
HLA-B*15:18 Japanese
HLA-B*59:01 Japanese
HLA-A*31:01 Northern European
HLA-A*31:01 Japanese, Korean
DRESS HLA-A*31:01 European, Japanese, Chinese
Oxcarbazepine SJS/TEN HLA-B*15:02, HLA-B*15:18 Han Chinese
Phenytoin SJS/TEN HLA-B*15:02 Han Chinese, Thai
HLA-B*13:01 Han Chinese
HLA-C*08:01/DRB1*16:02 Han Chinese
SJS/TEN/DRESS CYP2C9*3 Taiwan, Japan, Malaysia
Phenobarbital SJS/TEN ?HLA-B*51:01 Japan
Lamotrigine SJS/TEN HLA-B*15:02
HLA-B38
HLA-A*68:01
Han Chinese
Zonisamide SJS/TEN ?HLA-A*02:07 Japan
*HLA-B*15:21 is an important association with carbamazepine SJS/TEN.
TABLE XCIX. Valproic acid oral desensitization protocol*
Interval (d) Solution† Volume (mL) Daily dose (mg)
1-2 A 0.5 0.05
3-4 A 1 0.1
5-6 A 2.5 0.25
7-8 A 5 0.5
9-10 A 7.5 0.75
11-12 B 0.2 1
13-14 B 0.5 2.5
15-16 B 1 5
17-18 B 2 10
19-20 B 5 25
21-22 C 0.25 50
23-24 C 0.5 100
25-26 1 tablet — 200
27-28 1 tablet twice a day — 400
29-30 2 tablets twice a day — 800
*As adapted by Toker et al.
881
†Solution A: The solution was made by 1:50 dilution of solution B to a final concentration of 0.1 mg/mL. Solution B: The solution was prepared by diluting 2 mL of valproic
acid solution (Depalept Oral Solution-CTS; 200 mg/mL) into a volume of 78 mL diluent (water) to a final concentration of 5 mg/mL. Solution C: Valproic acid oral solution
(Depalept Oral Solution-CTS; 200 mg/mL). Valproic acid tablet (Depalept-CTS; 200 mg).
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S93

anticonvulsant drug users. Immediate IgE-mediated reactions
such as urticaria or angioedema are rarely described as compared
with the DHR.
Major symptoms of hypersensitivity. The term “anti-
convulsant hypersensitivity syndrome” has been used to encap-
sulate the triad of fever, rash, and internal organ involvement,
but other features such as lymphadenopathy and eosinophilia are
commonly present.
855
This is likely a form of DRESS when
anticonvulsant therapy is implicated. In early literature, the
clinical cross-reactivity between aromatic amines such as
phenytoin, carbamazepine, and phenobarbital was as high as
75% as ascertained by positive rechallenge reactions.
855
In Eu-
ropean populations, an estimate of the higher end of risk of this
syndrome is 4.5/10,000. Later studies have shown that many
severe IM-ADRs associated with aromatic antiepileptic drugs are
associated with specific HLA alleles; however, even before these,
case reports suggested a familial predisposition and 10- to 1000-
fold overrepresentation of some of the IM-ADRs in specific
ethnicities in which the risk HLA alleles are prevalent.
353,856-858
Immediate-type, IgE-mediated symptoms have been described
in the literature. Case reports have described urticaria and/or
angioedema to oxcarbazapine in 9 pediatric patients, to pheno-
barbital in 1 pediatric patient, to carbamazepine in an adult, and
tongue angioedema to phenytoin in a child.
859,860
Anaphylaxis is
rarely reported. It is important to be aware that immediate-type
symptoms may occur, but these are not as frequent as delayed-
type drug hypersensitivity reactions. Many of these case reports
cannot be validated as truly mediated by IgE. Also, angioedema
or other benign exanthem may be misdiagnosed as IgE-mediated
when in fact it may be the presenting cutaneous manifestation of
anticonvulsant syndrome.
One study examining different appearances of rashes associ-
ated with anticonvulsants reported that aromatic anticonvulsants
are significantly associated with IgE-mediated (immediate) type I
and cell-mediated (nonimmediate or delayed) type IV drug
TABLE C. Desensitization protocol for pentobarbital
877,880
Desensitization strategy
Pentobarbital 0.25
m
g/kg ____ kg ¼ ____
m
g in 5 mL normal saline (NS) intravenously (IV) 1 over 30 min, followed immediately by
Pentobarbital 2.5
m
g/kg ____kg ¼ ____
m
g in 5 mL NS IV 1 over 30 min, followed immediately by
Pentobarbital 25
m
g/kg ____kg ¼ ____
m
g in 5 mL NS IV 1 over 30 min, followed immediately by
Pentobarbital 250
m
g/kg ____kg ¼ ____
m
g in 5 mL NS IV 1 over 30 min, followed immediately by
Pentobarbital 500
m
g/kg ____kg ¼ ____
m
g in 5 mL NS IV 1 over 30 min, followed immediately by
Pentobarbital 10 mg/kg ____kg ¼ ____
m
g in 5 mL NS IV 1 over 120 min, followed immediately by the full therapeutic starting dose
Full therapeutic starting dose: Must be continued on schedule without interruption
Pentobarbital 1 mg/kg/h ____kg ¼ ____ mg/h IV continuous solution
TABLE CI. Intravenous iron preparations*
Drug Trade name Available in the United States Test dose TDI* FDA boxed warning
HMW-ID DexFerrum; Imferon N (discontinued) Y Y Y
LMW-ID InFeD Y Y Y Y
Ferric gluconate Ferrlecit Y N N N
Iron sucrose Venofer Y N N N
Ferumoxytol Feraheme Y N Y Y
Iron isomaltoside Monofer Y N Y N
Ferric carboxymaltose Injectafer Y N N N
ID, Iron dextran; LMW, low molecular weight; N, no; TDI, total dose infusion; Y, yes.
*Allowing single dose administration of a patient’s entire iron requirement, rather than needing multiple smaller doses.
TABLE CII. Treatment of parenteral iron reactions
Mild Arthralgias, myalgias, flushing, mild chest tightness,
mild hypotension, nausea, itching
Pause infusion until symptoms resolve, then resume at 25%-50% rate.
Consider pausing and giving a steroid if recurs. Observe 60 min after
completion of infusion
Mild with urticaria As above, with urticaria Pause infusion, give antihistamine or steroid. Observe until symptoms
subside, then resume infusion
Moderate Severe chest pain, cough, nausea, tachycardia,
hypotension
Stop infusion. Give IV fluids, steroids, and antihistamines. Consider
alternative agent
Severe Bronchospasm, stridor, hypoxemia, significant
hypotension, tachycardia, angioedema
Stop infusion. Give epinephrine, oxygen, inhaled
b
2
-agonists, IV fluids,
steroids, antihistamines, other resuscitation
Delayed Arthralgias, myalgias, flushing Corticosteroid premedication
NSAIDs for symptomatic treatment
IV, Intravenous.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S94 BROYLES ET AL

hypersensitivity reaction, with a reported odds ratio of 2.15 and
6.06, respectively. The caveat, however, was that clinical phe-
notypes were not validated by additional formal causality
assessment or additional diagnostic (eg, skin) testing. The aro-
matic amine anticonvulsants have been more signi ficantly asso-
ciated with delayed cutaneous reactions.
861
Pharmacogenetic studies. The aromatic amine anticon-
vulsants share an aromatic benzene ring and are metabolized by
SMZ-TMP enzymes to hydroxylated aromatic amine com-
pounds (arene oxides). It was initially hypothesized that reactive
metabolites of these drugs haptenated cellular proteins leading to
an antigen-specific response. More recently, cellular models have
supported HLA-restricted, dose-dependent, and noncovalent
interactions between these aromatic amine anticonvulsant drugs
and HLA molecules/T-cell receptor. The genetic associations
have been strongest for HLA serotype B75 alleles, such as HLA-
B*15:02 and carbamazepine SJS/TEN, which are more prevalent
in Southeast Asian populations. Since 2007, the FDA has rec-
ommended HLA-B*15:02 screening for all patients of known or
suspected Southeast Asian ancestry before initiating carbamaze-
pine treatment. A prospective 1-arm study from Taiwan has
supported a reduction in the incidence of carbamazepine SJS/
TEN in association with HLA-B*15:02 screening.
862,863
Another study from Hong Kong showed that mandated HLA-
B*15:02 screening did not result in a reduction in the incidence
of SJS/TEN overall. This is because HLA-B*15:02 testing
appeared to lead to displacement of carbamazepine prescribing
with the prescription of phenytoin and a subsequent increase in
phenytoin SJS/TEN, thus underscoring the fact that provider
engagement and education is often necessary for clinical trans-
lation.
864
HLA risk alleles have had a low (<5%) positive pre-
dictive value for the development of IM-ADRs associated with
antiepileptic drugs, suggesting that other factors are important in
the immunopathogenesis. More recently, a dominant T-cell re-
ceptor clonotype has been found from blister fluid and PBMCs
in patients with acute- and recovery-phase carbamazepine SJS/
TEN.
353
Testing and diagnosis. The utility and cost-effectiveness of
HLA screening is currently largely based on the population
prevalence of disease carriage rate of the specific HLA allele in the
population in question and the positive predictive value of the
HLA risk allele for the development of the IM-ADR. The
number needed to treat to prevent 1 case of IM-ADR can vary
from a few hundred to more than 10,000.
353,857
Cost-effec-
tiveness decisions, however, are also driven by the fact that SJS/
TEN, the most severe of IM-ADRs associated with these aro-
matic anticonvulsants, has significant long-term morbidity and
mortality of up to 50% or higher, particularly in aging pop-
ulations.
865-867
The prevalence of HLA-B*15:02 is 7% to 15%
or higher in Southeast Asia and India but less than 1% in
populations of European and African ancestry.
353
HLA-A*31:01
has been associated with carbamazepine exanthem (mac-
ulopapular eruption) and DRESS in multiple ethnicities
including European. However, the generalizability of an associ-
ation between HLA-A*31:01 and carbamazepine SJS/TEN needs
to be further clarified.
353,858,865,867,868
Lamotrigine has also been
associated with a risk of severe immunologically mediated drug
reactions, though it is primarily glucuronidated rather than
oxidized like the other aromatic anticonvulsants.
855
Graded dose
introduction has been an effective way to reduce the incidence of
isolated drug eruptions associated with lamotrigine. Valproic acid
and newer structurally disparate anticonvulsants have been safe
to administer in patients with reactions to the aromatic antiep-
ileptic drugs. However, the risk of a cutaneous reaction is
increased with concurrent use of valproic acid and lamotrigine
because of metabolic interactions that lead to an increased
elimination half-life of lamotrigine and accumulation of the
parent drug.
869
In vivo testing such as patch testing has been used successfully
in such studies, showing sensitivity of more than 80%, particu-
larly in carbamazepine-associated DRESS in some studies, but
significantly less than 50% in others. Overall sensitivity for patch
testing with antiepileptic drugs varies from less than 20% to
more than 80%.
52,870,871
Patch tests should be left in situ for 48
hours and ideally read at 48 hours, 72 hours, 96 hours, and 1
week. The vehicle and concentration appear to be important, and
TABLE CIII. Sample 12-step rapid desensitization protocol for ferric gluconate
Solution (100 mL each) Concentration (mg/mL) mg/bag Volume infused (mL)
1 0.0125 1.25 9.25
2 0.125 12.5 18.75
3 1.2254 122.5 100
Step Solution Rate (mL/h) Time (min) Volume (mL) Dose (mg) Cumulative dose (mg)
1 1 2 15 0.5 0.0063 0.0063
2 1 5 15 1.25 0.0156 0.0219
3 1 10 15 2.5 0.0313 0.0531
4 1 20 15 5 0.0625 0.1156
5 2 5 15 1.25 0.1563 0.2719
6 2 10 15 2.5 0.3125 0.5844
7 2 20 15 5 0.6250 1.2094
8 2 40 15 10 1.2500 2.4594
9 3 10 15 2.5 3.0635 5.5229
10 3 20 15 5 6.1270 11.6499
11 3 40 15 10 12.2541 23.9040
12 3 80 61.875 82.5 101.0960 125.0000
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S95
sensitivity is highest in DRESS>AGEP>SJS/TEN.
52
For patch
testing, it is recommended to use between 1% and 10% (wt/wt)
of pure drug or 30% (wt/wt) concentration of the powdered
commercial tablet when the pure drug form cannot be patch
tested. Variability in positive results of patch testing with varying
concentrations across different anticonvulsants signifies the need
for patch testing with several concentrations for accurate results,
most commonly 1%, 10%, and 30% of the various antiepileptics
in petrolatum.
52,871,872
Carbamazepine patch testing appears to
be associated with the highest sensitivity, and some studies have
been able to show a significant cross-reactivity on patch testing
between drugs such as carbamazepine and phenytoin.
52,871,872
The genetic significance of this is still not known because there is
a much weaker association between phenytoin SJS/TEN and
HLA-B*15:02. From a recent genome-wide association study
that included populations from Taiwan, Malaysia, and Japan
with phenytoin SJS/TEN/DRESS, a strong association was
identified with a poor metabolizing genotype of the primary
oxidizing enzyme of phenytoin, CYP2C9 (CYP2C9*3), and not
significantly with genes in the HLA region.
873-875
One study
showed greater sensitivity of delayed prick and intradermal
testing compared with patch testing, but this has not been uni-
versally applied.
52
The lack of commercial availability of sterile
intravenous solutions for the aromatic anticonvulsants has
limited the use of intradermal testing for anticonvulsant hyper-
sensitivity.
52
In addition, prick and intradermal testing have been
avoided in very severe reactions such as SJS/TEN because of the
rare instances of systemic reactions. Ex vivo and in vitro tests such
as ELISpot, lymphocyte toxicity assay, and lymphocyte trans-
formation tests for the aromatic anticonvulsant drugs have been
used in the research setting. They currently lack sufficient posi-
tive and negative predictive value and have not been quality
assured for clinical use.
353,855,857,876
True immediate reactions (IgE-mediated) to antiepileptic
drugs including the aromatic amine anticonvulsants are very
uncommon, and validated skin testing protocols for immediate
hypersensitivity reactions to antiepileptics are hence not avail-
able. One case study has described successful skin testing to
phenobarbital and pentobarbital. For skin testing, 0.1 mg/mL
and 1 mg/mL concentrations were nonirritating in both the
patient and control and may be considered.
877
Caution is also
advised, because on second exposure to the implicated anticon-
vulsant drug or a structurally related aromatic amine anticon-
vulsant, a reaction that is actually T-cellemediated can appear
immediate or accelerated in nature because it is a second-expo-
sure memory T-cell response. These reactions are mechanistically
distinct from IgE-mediated reactions and noneIgE-mediated
mast cell activation. Many are potentially severe reactions that
can be life-threatening with continued dosing of the drugs.
Management. Many patients with hypersensitivity to one
aromatic anticonvulsant may experience similar symptoms with
other cross-reactive aromatic anticonvulsants, but there are
alternative agents without this structural similarity. Desensitiza-
tion and slow reintroduction protocols exist and are aimed to-
ward patients with histories of delayed reactions including mild
rashes without accompanying fever, internal organ, mucosal, or
severe skin involvement.
878,879
SCAR hypersensitivity reactions
should result in discontinuation of the offending drug, and
desensitization protocols should not be performed on these pa-
tients. There are a few published desensitization protocols in the
literature to both oxcarbazepine and valproic acid following mild-
delayed rashes (Table XCIX).
877,878,880,881
Both an oral desen-
sitization to phenobarbital and an intravenous desensitization to
pentobarbital have been reported to be successful in cases of
immediate hypersensitivity (Table C).
877,880
Benzodiazepines (by Parul Kothari, MD)
General.
Benzodiazepines are prescribed for a wide variety of
disorders, including anxiety, seizures, insomnia, muscle spasms,
and alcohol withdrawal. They act in the central nervous system
to enhance the inhibitory effect of
g
-aminobutyric acid. Because
they have the potential to be abused, they are controlled sub-
stances regulated by the US Drug Enforcement Agency. They are
commonly classified as short-, intermediate-, or long-acting, on
the basis of their half-life.
Structurally, benzodiazepines are composed of benzene and
diazepine rings that are fused together. Benzodiazepines differ by
their side chains, which can alter their specific pharmacologic
properties. Among the structural differences, alprazolam has a
unique triazole ring and tetrazepam a cyclohexene ring.
Major symptoms of hypersensitivity. Allergic reactions
to benzodiazepines are rare, and most of the literature is limited
to case reports and small case series.
882-887
Both immediate and
delayed reactions have been reported to range in severity from
mild to severe, including SJS and TEN. A recent cohort study
estimated the cumulative incidence of SJS/TEN to benzodiaze-
pines to be 3.76 per million new users.
886
The most common
benzodiazepine implicated in the literature to date has been
tetrazepam, which is not available in the United States.
Diagnosis. Although diagnostic testing for hypersensitivity
reactions to benzodiazepines is not validated, there are reports in
the literature of skin and patch testing to support the diagnosis.
Currently, the determination of a benzodiazepine allergy relies
on a careful history with attention to objective findings at the
time of the reaction. For midazolam, the maximum nonirritating
concentration for skin testing has been reported as 1 to 5 mg/mL
and 0.25 to 0.5 mg/mL for PTs and IDTs, respectively.
79,888
Management. Given the rarity of allergic reactions to ben-
zodiazepines, there are no studies that have evaluated cross-
reactivity among members of this class of medication. In a case
report of an IgE-mediated hypersensitivity reaction to tetraze-
pam, the patient had a negative skin test result and tolerated a
challenge to diazepam.
885
In a case series of delayed cutaneous
reactions to tetrazepam, all 9 subjects were tolerant to other
benzodiazepines, as demonstrated by negative testing result and/
or challenge
.883
This was attributed to differences between the
structure of tetrazepam and other benzodiazepines. However,
others have reported cross-reactivity between tetrazepam and
diazepam as well as contact dermatitis to multiple benzodiaze-
pines.
884,889
If an alternative benzodiazepine is required, one that
has been previously tolerated should be used and/or a supervised
oral challenge performed. Currently, there are no desensitization
protocols for benzodiazepines in the literature.
Muscarinic antagonists (by Samantha Minnicozzi,
MD)
General.
Muscarinic antagonists are drugs that bind to
muscarinic receptors and inhibit acetylcholine from binding and
its subsequent downstream effects. For example, atropine is used
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S96 BROYLES ET AL
for the treatment of childhood myopia and for pupillary dilation
and bradycardia, and as an antidote for cholinergic toxicity.
Scopolamine is used to prevent nausea during chemotherapy
regimens and postoperatively. Glycopyrrolate decreases secre-
tions, and is commonly used preoperatively and perioperatively
for airway management. Oxybutynin is used to treat patients
with bladder difficulties including urge and incontinence.
All antimuscarinic agents have wide side-effect profiles
depending on which muscarinic receptor is targeted. Common
side effects of antimuscarinic agents include mydriasis, dry
mouth, tachycardia, agitation, delirium, and hyperthermia. In-
fants and children are particularly susceptible to these hyper-
thermic effects.
A review of topical atropine use found a 1% to 4% risk of
minor reactions, with more severe effects observed with intra-
venous administration including cardiac arrhythmias.
890,891
Hypersensitivity reactions have rarely been reported, with very
few case reports describing atropine and cyclopentolate causing
suspected IgE-mediated reactions.
892-894
Major symptom s of hypersensitivity. Among the anti-
muscarinic drugs, atropine has the largest accumulation of case
reports describing anaphylaxis to parenteral and topical formu-
lations. In a review of atropine applied topically in pediatric
patients for the treatment of myopia, 3.2% developed allergic
conjunctivitis, with 0.8% experiencing an allergic dermatitis.
890
In case reports of suspected atropine and cyclopentolate,
anaphylaxis symptoms included generalized urticaria, facial and
eyelid edema, pruritus, vomiting, and hypotension.
892-895
Delayed hypersensitivities and contact allergies to anti-
muscarinic agents are another drug-related allergy. In a large
study evaluating the safety of topical atropine for the treatment of
myopia in children, up to 0.8% developed a contact allergy.
890
Oral oxybutynin has been implicated in a case of fixed drug
eruption that continued intermittently over months, coinciding
with the use of oxybutynin, and resolved only once therapy was
discontinued.
896
Inhaled antimuscarinic agents are a mainstay in the treatment
of COPD. A review of the literature on either asthma or COPD
found no case reports of history of inhaled agents such as ipra-
tropium or tiotropium causing immediate hypersensitivity re-
actions. There was a single case report of a patient with COPD
who developed an intermittent whole-body pruritic rash for
several years.
897
A biopsy of the rash was consistent with a drug
eruption. The patient was initially given a tiotropium inhaler,
which was discontinued. Following discontinuation, the patient
was started on an ipratropium metered-dose inhaler, after which
the rash recurred 3 days later and disappeared 5 days after dis-
continuing therapy. The patient continued to develop a similar
rash waxing and waning with the use of umeclidinium and
aclidinium until all inhaled antimuscarinic therapy was
discontinued.
897
Diagnosis. Skin testing for the evaluation of immediate hy-
persensitivity to antimuscarinic agents has not been commonly
performed and has not been validated on review of the literature.
Cavanah and Casale
898
describe antimuscarinic intradermal skin
testing to atropine and scopolamine in 7 healthy volunteers using
volumes of 20 nmol and 2 nmol. The solutions used for testing
in this protocol were created by diluting either atropine or
scopolamine in 0.9% saline, 0.03% human albumin, and 0.4%
phenol in water and then buffering the solution to a pH of 6.5 to
7.5. All subjects developed a wheal-and-flare reaction to atropine
at both doses.
898
In comparison, all subjects reacted with a
wheal-and-flare response to 20 nmol of scopolamine and none
had a response to 2 nmol.
898
Fisher and Bowey
899
performed atropine skin testing as part of
their perioperative anaphylaxis evaluation in 10 individuals. They
performed skin prick testing using undiluted atropine at a con-
centration of 0.6 mg/mL and intradermal testing at a dilution of
1:1000.
899
Similarly, Cabrera-Freitag et al
892
performed skin
prick and intradermal drug testing to atropine to evaluate a case
of anaphylaxis. Atropine was used at a concentration of 1 mg/mL
for skin prick testing and a dilution of 0.1 mg/mL for intra-
dermal testing. The patient had a positive result on intradermal
testing, with negative intradermal testing result in 10 control
subjects.
892
Management. There is little evidence in the literature
regarding cross-reactivity in patients with reported allergy to one
antimuscarinic agent and their ability to tolerate other anti-
muscarinic agents. The case reports discussing allergic reactions
recommend avoidance of similar antimuscarinic drugs in the
future and do not discuss class alternatives. Desensitization
protocols to antimuscarinic agents are yet to be described.
Iron (by Anne Liu, MD)
General.
Intravenous iron is an essential aspect of iron-
deficiency anemia when side effects or absorption limit the use of
oral formulations. Intravenous iron preparations are colloids of
iron-carbohydrate nanoparticles that vary by iron core size and
type of surrounding carbohydrate, processed and released as free
labile iron.
Formulations available in the United States include low-mo-
lecular-weight iron dextran, ferric gluconate, iron sucrose, fer-
umoxytol, iron isomaltoside, and ferric carboxymaltose
(Table CI). High-molecular-weight (HMW) iron dextrans were
discontinued in the United States.
Hypersensitivity reactions. Labile plasma iron may pro-
duce nonspecific oxidative stress and complement activa-
tion.
900,901
Excluding HMW iron dextrans, evidence for
immune complex formation and IgE-mediated mechanisms is
paltry, and tryptase elevation is exceedingly rare.
902,903
Adverse reactions may be infusion rateedependent and typi-
cally manifest as dyspnea, chest and/or back pain, acute ar-
thralgias and myalgias, hypotension, tachycardia, nausea/
vomiting, and pruritus.
904-906
Minor reactions usually abate
without treatment or with rate reduction and may not recur on
rechallenge.
905
Angioedema and urticaria may signal a distinct
reaction type. Severe reactions including anaphylaxis can be fatal.
Rarely, delayed reactions may exhibit hypotension, arthralgias/
myalgias, malaise, and vomiting. Iron sucrose can uniquely cause
peripheral edema and renal injury in a rate- and concentration-
related manner.
906,907
Excluding HMW iron dextrans, incidence of serious adverse
reactions is less than 1 in 200,000, accounting for approximately
0 to 5 deaths per year in the United States.
906,908
The high re-
action rate and mortality associated with iron dextrans has been
attributed to the HMW iron dextran formulation.
908-910
Limited
data suggest iron isomaltoside carries a higher reaction risk than
ferric carboxymaltose.
911
Significant differences in reaction risk
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S97
have not been shown among low-molecular-weight iron dextran,
iron sucrose, ferric gluconate, and ferric carboxymaltose.
908,912-917
Adverse event reporting initially suggested an increased risk of
fatal reactions from ferumoxytol, but randomized comparisons of
ferumoxytol with iron sucrose and ferric carboxymaltose reported
similar rates of adverse reactions.
918-921
Risk factors for severe hypersensitivity reactions include atopy
and multiple drug allergies including previous iron hypersensi-
tivity reactions.
900,905,922
Diagnosis. Evaluation of an iron reaction should document
the specific product and dose, route, and rate of administration,
and include a detailed description of the reaction, treatment, and
response.
Graded challenge is required only for low-molecular-weight
iron dextrans and may not predict serious hypersensitivity re-
actions.
922
Skin testing has limited utility because most reactions
are not IgE-mediated, but it may detect a subset of patients who
are sensitized via typical allergic pathways. A protocol for ferric
gluconate skin testing has been used: skin prick at 12.5 mg/mL,
and IDT at 0.0125, 0.125, and 1.25 mg/mL at the Brigham and
Women’s Hospital. Skin testing to iron sucrose should not be
performed, because a permanent tattoo can form from deposition
of iron pigment.
Management. Table CII outlines suggested reaction treat-
ments based on expert panel consensus.
916,922
Premedication
with antihistamines is not advised because of ineffectiveness and
side effects.
916,922
Switching agents can be considered in patients
with mild to moderate reactions, but expert panels advise
avoiding all parenteral iron after a severe reaction.
916
Ferric
gluconate and iron sucrose, but not ferumoxytol, are considered
alternatives for patients sensitive to iron dextran.
902,923,924
Desensitization to HMW iron dextrans has been re-
ported.
925,926
Desensitization protocols to ferric carboxymaltose
and iron sucrose have been reported in patients with a history of
anaphylaxis to other iron products.
927,928
In the Brigham and
Women’s Hospital experience, allergists have successfully per-
formed ferric gluconate desensitizations (Table CIII). Desensi-
tization to iron dextrans has been difficult to complete because of
treatment-refractory reactions. Iron sucrose desensitization is not
done because of poor stability at lower concentrations. Further
data are needed to elucidate a role for rapid desensitization after
parenteral iron anaphylaxis.
Acknowledgments
We thank Dawn Angel, Becky Drager, Margaret Lemay,
Beena Rao, and Erin Scott for their invaluable medical editorial
assistance with this manuscript and Michael Schatz for his
steadfast encouragement and support of this project
REFERENCES
1. Broyles AD, Banerji A, Castells M. Practical guidelines for the evaluation and
management of drug hypersensitivity: general concepts. J Allergy Clin
Immunol Pract 2020;8:S3-15.
2. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr
Opin Allergy Clin Immunol 2005;5:309-16.
3. Solensky R. Allergy to
b
-lactam antibiotics. J Allergy Clin Immunol 2012;130:
1442.e5.
4. Macy E. Routine penicillin skin testing in hospitalized patients with a history
of penicillin allergy. Perm J 2004;8:20-4.
5. Lee CE, Zembo wer TR, Fotis MA, Postelnick MJ, Greenberger PA,
Peterson LR, et al. The incidence of antimicrobial allergies in hospitalized
patients. Arch Intern Med 2000;160:2819.
6. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic
treatment of penicillin-allergic patients in internal medicine wards of a general
tertiary care hospital. Clin Exp Allergy 2003;33:501-6.
7. Macy E, Contreras R. Health care use and serious infection prevalence asso-
ciated with penicillin “allergy” in hospitalized patients: a cohort study.
J Allergy Clin Immunol 2014;133:790-6.
8. Macy EM, Ngor E. Safely diagnosing clinically significant penicillin allergy
with only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy
Clin Immunol 2013;131:AB234.
9. del Real GA, Rose ME, Ramirez-Atamoros MT, Hammel J, Gordon SM,
Arroliga AC, et al. Penicillin skin testing in patients with a history of
b
-lactam
allergy. Ann Allergy Asthma Immunol 2007;98:355-9.
10. Blanca M, Torres MJ, García JJ, Romano A, Mayorga C, de Ramon E, et al.
Natural evolution of skin test sensitivity in patients allergic to
b
-lactam anti-
biotics. J Allergy Clin Immunol 1999;103:918-24.
11. Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to
detect penicillin allergy. J Allergy Clin Immunol 1981;68:171-80.
12. Kerns D, Shira JE, Go S, Summers RJ, Schwab JA, Plunket DC. Ampicillin
rash in children: relationship to penicillin allergy and infectious mono-
nucleosis. Am J Dis Child 1973;125:187-90.
13. Joint Task For ce on Practice Para meters; Amer ican Academ y of Allergy,
Asthma and Imm unology; American College o f Allergy, Asthma and
Immunology; Joint Council of Allergy Asthma and Imm unology. Drug
allergy: an updated practice parameter. Ann Allergy Asthma Immunol
2010;105:259-273.e7 8.
14. Mirakian R, Leech SC, Krishna MT, Richter AG, Huber PAJ, Farooque S,
et al. Managemen t of allergy to penicillins and other beta-lactams. Clin Exp
Allergy 2015;45:300-27.
15. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al.
Update on the evaluation of hypersensitivity reactions to betalactams. Allergy
2009;64:183-93.
16. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA,
et al. International Consensus on drug allergy. Allergy 2014;69:420-37.
17. Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and
management of drug hypersensitivity reactions. J Allergy Clin Immunol 2011;
127:S67-73.
18. Bircher AJ, Scherer Hofmeier K. Drug hypersensitivity reactions: inconsis-
tency in the use of the classification of immediate and nonimmediate reactions.
J Allergy Clin Immunol 2012;129:263-4.
19. Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med 2003;
139:683.
20. Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, et al.
Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy 2004;
59:1153-60.
21. Solensky R. Hypersensitivity reactions to beta-lactam antibiotics. Clin Rev
Allergy Immunol 2003;24:201-20.
22. Feingold DS, Parris EE, Levine BB. Immunologic mechanisms of penicillin
allergy. N Engl J Med 1966;275:1115-25.
23. Torres MJ, Blanca M, Fernandez J, Romano A, Weck A, Aberer W, et al.
Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy
2003;58:961-72.
24. Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with
penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:
2456-63.
25. Geng B, Eastman JJ, Mori K, Braskett M, Riedl MA. Utility of minor de-
terminants for skin testing in inpatient penic illin allergy evaluation. Ann Al-
lergy Asthma Immunol 2017;119:258-61.
26. Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a
determinant in penicillin skin testing. Int Arch Allergy Immunol 2010;152:
313-8.
27. Macy E, Burchette RJ. Oral antibiotic adverse reactions after penicillin skin
testing: multi-year follow-up. Allergy 2002;57:1151-8.
28. Rosenfield L, Kalicinsky C, Warrington R. A retrospective comparison of false
negative skin test rates in penicillin allergy, using pencilloyl-poly-lysine and
minor determinants or Penicillin G, followed by open challenge. Allergy
Asthma Clin Immunol 2015;11:34.
29. Sogn DD, Evans R III, Shepherd GM, Casale TB, Condemi J, Greenberger PA,
et al. Results of the National Institute of Allergy and Infectious Diseases
Collaborative Clinical Trial to test the predictive value of skin testing with
major and minor penicillin derivatives in hospitalized adults. Arch Intern Med
1992;152:1025-32.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S98 BROYLES ET AL
30. Solensky R, Jacobs J, Lester M, Lieberman P, McCafferty F, Nilsson T, et al.
Penicillin allergy evaluation: a prospective, multicenter, open-label evaluatio n
of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract 2019;
7:1876-1885.e3.
31. Confino-Cohen R, Rosman Y, Lachover I, Meir Shafrir K, Goldberg A. The
importance of amoxicillin and amoxicillin-clavulanate determinants in the
diagnosis of immediate allergic reactions to beta-lactams. Int Arch Allergy
Immunol 2016;170:62-6.
32. Atanaskovic-Markovic M, Velickovic TC, Gavrovic-Jankulovic M,
Vuckovic O, Nestorovic B. Immediate allergic reactions to cephalosporins and
penicillins and their cross-reactivity in children. Pediatr Allergy Immunol
2005;16:341-7.
33. Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and
cephalosporin allergy: reliability of examination assessed by skin testing and
oral challenge. J Pediatr 1998;132:137-43.
34. Ponvert C, Weilenmann C, Wassenberg J, Walecki P, Bourgeois ML, de
Blic J, et al. Allergy to betalactam antibiotics in children: a prospective follow-
up study in re-treated children after negative responses in skin and challenge
tests. Allergy 2007;62:42-6.
35. Iglesias-Souto J, Gonzalez R, Poza P, Sanchez-Machin I, Matheu V. Evalu-
ating the usefulness of retesting for beta-lactam allergy in children. Pediatr
Infect Dis J 2012;31:1091-3.
36. Macy E. Penicillin skin testing in pregnant women with a history of penicillin
allergy and group B streptococcus colonization. Ann Allergy Asthma Immunol
2006;97:164-8.
37. Blanca M , Mayorga C, Torre s MJ, Reche M, Moy a MC, Rodriguez JL,
et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amox-
icilloyl and benzylpenicillo yl in patients with p enicillin allergy. Allergy
2001;56:862-70.
38. Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, et al.
Relevance of the determination of serum-specific IgE antibodies in the diag-
nosis of immediate beta-lactam allergy. Allergy 2007;62:47-52.
39. Sanz ML, Gamboa PM, Antepara I, Uasuf C, Vila L, Garcia-Aviles C, et al.
Flow cytometric basophil activation test by detection of CD63 expression in
patients with immediate-type reactions to betalactam antibiotics. Clin Exp
Allergy 2002;32:277-86.
40. Torres MJ, Romano A, Mayorga C, Moya MC, Guzman AE, Reche M, et al.
Diagnostic evaluation of a large group of patients with immediate allergy to
penicillins: the role of skin testing. Allergy 2001;56:850-6.
41. Torres MJ, Padial A, Mayorga C, Fernandez T, Sanchez-Sabate E, Cornejo-
Garcia JA, et al. The diagnostic interpreta tion of basophil activation test in
immediate allergic reactions to betalactams. Clin Exp Allergy 2004;34:
1768-75.
42. Macy E. Penicillin allergy: optimizing diagnostic protocols, public health
implications, and future research needs. Curr Opin Allergy Clin Immunol
2015;15:308-13.
43. Hershkovich J, Broides A, Kirjner L, Smith H, Gorodischer R. Beta lactam
allergy and resensitization in children with suspected beta lactam allergy. Clin
Exp Allergy 2009;39:726-30.
44. Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need:
multiyear follow-up in 568 test result-negative subjects exposed to oral peni-
cillins. J Allergy Clin Immunol 2003;111:1111-5.
45. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in pa-
tients with a history of penicillin allergy after receiving repeated penicillin
courses. Arch Intern Med 2002;162:822-6.
46. Parker PJ, Parrinello JT, Condemi JJ, Rosenfeld SI. Penicillin resensitization
among hospitalized patients. J Allergy Clin Immunol 1991;88:213-7.
47. Dorman SM, Seth S, Khan DA. Risk of allergic reactions to recurrent intra-
venous penicillin administration in penicillin skin test negative patients.
J Allergy Clin Immunol Pract 2018;6:196-200.
48. Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. The very
limited usefulness of skin testing with penicilloyl-polylysine and the minor
determinant mixture in evaluating nonimmediate reactions to penicillins. Al-
lergy 2010;65:1104-7.
49. Padial A, Antunez C, Blanca-Lopez N, Fernandez TD, Cornejo-Garcia JA,
Mayorga C, et al. Non-immediate reactions to beta-lactams: diagnostic
value of skin testing and drug provocation test. Clin Exp Allergy 2008;38:
822-8.
50. Patriarca G, D’Ambrosio C, Schiavino D, Larocca LM, Nucera E, Milani A.
Clinical usefulness of patch and challenge tests in the diagnosis of
cell-mediated allergy to betalactams. Ann Allergy Asthma Immunol 1999;83:
257-66.
51. Romano A, Viola M, Mondino C, Pettinato R, Di Fonso M, Papa G, et al.
Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch
Allergy Immunol 2002;129:169-74.
52. Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M,
et al. A multicentre study to determine the value and safety of drug patch tests
for the three main classes of severe cutaneous adverse drug reactions. Br J
Dermatol 2013;168:555-62.
53. Pinho A, Coutinho I, Gameiro A, Gouveia M, Goncalo M. Patch testing—a
valuable tool for investigating non-immediate cutaneous adverse drug re-
actions to antibiotics. J Eur Acad Dermatol Venereol 2017;31:280-7.
54. Phillips EJ, Bigliardi P, Bircher AJ, Broyles A, Chang YS, Chung WH, et al.
Controversies in drug allergy: testing for delayed reactions. J Allergy Clin
Immunol 2019;143:66-73.
55. Borch JE, Bindslev-Jensen C. Full-course drug challenge test in the diagnosis
of delayed allergic reactions to penicillin. Int Arch Allergy Immunol 2011;155:
271-4.
56. Hjortlund
J, Mortz CG, Skov PS, Bindslev-Jensen C. Diagnosis of penicillin
allergy revisited: the value of case history, skin testing, specific IgE and pro-
longed challenge. Allergy 2013;68:1057-64.
57. Baldo BA. Penicillins and cephalosporins as allergens–structural aspects of
recognition and cross-reactions. Clin Exp Allergy 1999;29:744-9.
58. Blanca M, Vega JM, Garcia J, Carmona MJ, Terados S, Avila MJ, et al. Al-
lergy to penicillin with good tolerance to other penicillins: study of the inci-
dence in subjects allergic to beta-lactams. Clin Exp Allergy 1990;20:475-81.
59. Atanaskovic-Markovic M, Gaeta F, Gavrovic-Jankulovic M, Velickovic TC,
Valluzzi RL, Romano A. Tolerability of imipenem in children with IgE-
mediated hypersensitivity to penicillins. J Allergy Clin Immunol 2009;124:
167-9.
60. Atanaskovic-Markovic M, Gaeta F, Medjo B, Viola M, Nestorovic B,
Romano A. Tolerability of meropenem in children with IgE-mediated hyper-
sensitivity to penicillins. Allergy 2008;63:237-40.
61. Gaeta F, Valluzzi RL, Alonzi C, Maggioletti M, Caruso C, Romano A.
Tolerability of aztreonam and carbapenems in patients with IgE-mediated
hypersensitivity to penicillins. J Allergy Clin Immunol 2015;135:972-6 .
62. Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-
mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of
penicillins, monobactams, and carbapenems. J Allergy Clin Immunol 2010;
126:994-9.
63. Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL.
Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J
Med 2006;354:2835-7.
64. Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Valluzzi R, Gueant JL.
Brief communication: tolerability of meropenem in patients with IgE-mediated
hypersensitivity to penicillins. Ann Intern Med 2007;146:266-9.
65. Sodhi M, Axtell SS, Callahan J, Shekar R. Is it safe to use carbapenems in
patients with a history of allergy to penicillin? J Antimicrob Chemother 2004;
54:1155-7.
66. Saxon A, Swabb EA, Adkinson NF Jr. Investigation into the immunologic
cross-reactivity of aztreonam with other beta-lactam antibiotics. Am J Med
1985;78:19-26.
67. Vega JM, Blanca M, Garcia JJ, Miranda A, Carmona MJ, Garcia A, et al.
Tolerance to aztreonam in patients allergic to beta-lactam anti biotics. Allergy
1991;46:196-202.
68. Sanchez-Morillas L, Perez-Ezquerra PR, Reano-Martos M, Laguna-
Martinez JJ, Sanz ML, Martinez LM. Selective allergic reactions to clavulanic
acid: a report of 9 cases. J Allergy Clin Immunol 2010;126:177-9.
69. Torres MJ, Ariza A, Mayorga C, Dona I, Blanca- Lopez N, Rondon C, et al.
Clavulanic acid can be the component in amoxicillin-clavulanic acid respon-
sible for immediate hypersensitivity reactions. J Allergy Clin Immunol 2010;
125:502-505.e2.
70. Wendel GD Jr, Stark BJ, Jamison RB, Molina RD, Sullivan TJ. Penicillin
allergy and desensitization in serious infections during pregnancy. N Engl J
Med 1985;312:1229-32.
71. Castells M. Rapid desensitization for hypersensitivity reactions to medications.
Immunol Allergy Clin North Am 2009;29:585-606.
72. Caubet JC, Kaiser L, Lemaitre B, Fellay B, Gervaix A, Eigenmann PA. The
role of penicillin in benign skin rashes in childhood: a prospective study based
on drug rechallenge. J Allergy Clin Immunol 2011;127:218-22.
73. Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC,
Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients
reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol
2015;115:294-300.e2.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S99
74. Macy E, Contreras R. Adverse reactions associated with oral and parenteral use
of cephalosporins: a retrospective population-based analysis. J Allergy Clin
Immunol 2015;135:745-752.e5.
75. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence:
age and sex effects. Am J Med 2009;122:778.e1-e7.
76. Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS,
Walensky RP, et al. Peripheral blood eosinophilia and hypersensitivity re-
actions among patients receiving outpatient parenteral antibiotics. J Allergy
Clin Immunol 2015;136:1288-1294.e1.
77. Blumenthal KG, Shenoy ES, Wolfson AR, Berkowitz DN, Carballo VA,
Balekian DS, et al. Addressing inpatient beta-lactam allergies: a multihospital
implementation. J Allergy Clin Immunol Pract 2017;5:616-625.e7.
78. Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Zaffiro A, Caruso C, et al.
IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolera-
bility of alternative cephalosporins. J Allergy Clin Immuno l 2015;136:
685-691.e3.
79. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A,
Bilo MB, et al. Skin test concentrations for systemically administered drugs –
an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013;
68:702-12.
80. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin
test concentrations for commonly prescribed antibiotics. J Allergy Clin
Immunol 2003;112:629-30.
81. Romano A, Gaeta F, Valluzzi RL, Zaffiro A, Caruso C, Quaratino D. Natural
evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity
to cephalosporins. Allergy 2014;69:806-9.
82. Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schroder W,
Roujeau JC, et al. Correlations between clinical patterns and causes of ery-
thema mult iforme majus, Stevens-Johnson syndrome, and toxic epidermal
necrolysis: results of an international prospective study. Arch Dermatol 2002;
138:1019-24.
83. Blumenthal KG, Wickner PG, Hurwitz S, Pricco N, Nee AE, Laskowski K,
et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial
stewardship. J Allergy Clin Immunol 2017;140:154-61.e6.
84. Iammatteo M, Blumenthal KG, Saff R, Long AA, Banerji A. Safety and
outcomes of test doses for the evaluation of adverse drug reactions: a 5-year
retrospective review. J Allergy Clin Immunol Pract 2014;2:768-74.
85. Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions: a report
from the Boston Collaborative Drug Surveillance Program on 15,438
consecutive inpatients, 1975 to 1982. JAMA 1986;256:3358-63.
86. Cabanas R, Caballero MT, Vega A, Martin-Esteban M, Pascual C. Anaphy-
laxis to trimethoprim. J Allergy Clin Immunol 1996;97:137-8.
87. Jaffe HS, Abrams DI, Ammann AJ, Lewis BJ, Golden JA. Complications of
co-trimoxazole in treatment of AIDS-associated Pneumocystis carinii pneu-
monia in homosexual men. Lancet 1983;2:1109-11.
88. Kennedy CA, Pimentel JA, Lewis DE, Anderson MD, Weiss PJ,
Oldfield EC III. Crossover of human immunodeficiency virus-infected patients
from aerosolized pentamidine to trimethoprim-sulfamethoxazole: lack of he-
matologic toxicity and relationship of side effects to CD4þ lymphocyte count.
J Infect Dis 1993;168:314-7.
89. Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug
reactions in HIV infection. N Engl J Med 1993;328:1670-4.
90. Dibbern DA Jr, Montanaro A. Allergies to sulfonamide antibiotics and
sulfur-containing drugs. Ann Allergy Asthma Immunol 2008;100:
91-100.
91. Krantz MS, Stone CA Jr, Abreo A, Phillips EJ. Oral challenge with trimeth-
oprim-sulfamethoxazole in patients with “sulfa” antibiotic allergy. J Allergy
Clin Immunol Pract 2020;8:757-760.e4.
92. Khan DA, Knowles SR, Shear NH. Sulfonamide hypersensitivity: fact and
fiction. J Allergy Clin Immunol Pract 2019;7:2116-23.
93. Douglas R, Spelman D, Czarny D, O’Hehir RE. Successfu l desensitization
of two patients w ho previously developed Stevens-Johnson syndrome
while re ceiving trimethoprim-sulfame thoxazole. Clin I nfect Dis 1997;25 :
1480.
94. Absar N, Daneshvar H, Beall G. Desensitization to trimethoprim/sulfameth-
oxazole in HIV-infected patients. J Allergy Clin Immunol 1994;93:1001-5.
95. Caumes E, Guermonprez G, Lecomte C, Katlama C, Bricaire F. Efficacy and
safety of desensitization with sulfamethoxazole and trimethoprim in 48 pre-
viously hypersensitive patients infected with human immunodefi
ciency virus.
Arc
h Dermatol 1997;133:465-9.
96. Gluckstein D, Ruskin J. Rapid oral desensitization to trimethoprim-sulfa-
methoxazole (TMP-SMZ): use in prophylaxis for Pneumocystis carinii pneu-
monia in patients with AIDS who were previously intolerant to TMP-SMZ.
Clin Infect Dis 1995;20:849-53.
97. Kalanadhabhatta V, Muppidi D, Sahni H, Robles A, Kramer M. Successful
oral desensitization to trimethoprim-sulfamethoxazole in acquired immune
deficiency syndrome. Ann Allergy Asthma Immunol 1996;77:394-400.
98. Leoung GS, Stanford JF, Giordano MF, Stein A, Torres RA, Giffen CA, et al.
Trimethoprim-sulfamethoxazole (TMP-SMZ) dose escalation versus direct
rechallenge for Pneumocystis carinii pneumonia prophylaxis in human im-
munodeficiency virus-infected patients with previous adverse reaction to TMP-
SMZ. J Infect Dis 2001;184:992-7.
99. Mann R, Badesch D, Zamora M, Dreskin SC. Desensitization to trimethoprim-
sulfamethoxazole following lung transplantation. Chest 1997;111:1147.
100. Nguyen MT, Weiss PJ, Wallace MR. Two-day oral desensitization to
trimethoprim-sulfamethoxazole in HIV-infect ed patients. AIDS 1995;9:573-5.
101. Rich JD, Sullivan T, Greineder D, Kazanjian PH. Trimethoprim/sulfameth-
oxazole incremental dose regimen in human immunodeficiency virus-infected
persons. Ann Allergy Asthma Immunol 1997;79:409-14.
102. Ryan C, Madalon M, Wortham DW, Graziano FM. Sulfa hypersensitivity in
patients with HIV infection: onset, treatment, critical review of the literature.
WMJ 1998;97:23-7.
103. Straatmann A, Bahia F, Pedral-Sampaio D, Brites C. A randomized, pilot trial
comparing full versus escala ting dose regimens for the desensitization of AIDS
patients allergic to sulfonamides. Braz J Infect Dis 2002;6:276-80.
104. Yoshizawa S, Yasuoka A, Kikuchi Y, Honda M, Gatanaga H, Tachikawa N, et al.
A 5-day course of oral desensitization to trimethoprim/sulfamethoxazole (T/S) in
patients with human immunodeficiency virus type-1 infection who were previ-
ously intolerant to T/S. Ann Allergy Asthma Immunol 2000;85:241-4.
105. Soffritti S, Ricci G, Prete A, Rondelli R, Menna G, Pession A. Successful
desensitization to trimethoprim-sulfamethoxazole after allogeneic haemato-
poietic stem cell transplantation: preliminary observations. Med Pediatr Oncol
2003;40:271-2.
106. Moreno JN, Poblete RB, Maggio C, Gagnon S, Fischl MA. Rapid oral
desensitization for sulfonamides in patients with the acquired immunodefi-
ciency syndrome. Ann Allergy Asthma Immunol 1995;74:140-6.
107. White MV, Haddad ZH, Brunner E, Sainz C. Desensitization to trimethoprim
sulfamethoxazole in patients with acquired immune deficiency syndrome and
Pneumocystis carinii pneumonia. Ann Allergy 1989;62:177-9.
108. Pyle RC, Butterfield JH, Volcheck GW, Podjasek JC, Rank MA, Li JT, et al.
Successful outpatient graded administration of trimethoprim-sulfamethoxazole
in patients without HIV and with a history of sulfonamide adverse drug re-
action. J Allergy Clin Immunol Pract 2014;2:52-8.
109. Aranda A, Mayorga C, Ariza A, Dona I, Rosado A, Blanca-Lopez N, et al. In
vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones.
Allergy 2011;66:247-54.
110. Blanca-Lopez N, Ariza A, Dona I, Mayorga C, Montanez MI, Garcia-
Campos J, et al. Hypersensitivity reactions to fluoroquinolones: analysis of the
factors involved. Clin Exp Allergy 2013;43:560-7.
111. Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, et al. Fluo-
roquinolone-associated anaphylaxis in spontaneous adverse drug reaction re-
ports in Germany: differences in reporting rates between individual
fluoroquinolones and occurrence after first-ever use. Drug Saf 2006;29:
1087-100.
112. Seitz CS, Brocker EB, Trautmann A. Diagnostic testing in suspected fluo-
roquinolone hypersensitivity. Clin Exp Allergy 2009;39:1738-4 5.
113. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identi-
fication of a mast-cell-specific receptor crucial for pseudo-allergic drug re-
actions. Nature 2015;519:237-41.
114. Manfredi
M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, et al.
Detection of specific IgE to quinolones. J Allergy Clin Immuno l 2004;113:
155-60.
115. Venturini Diaz M, Lobera Labairu T, del Pozo Gil MD, Blasco Sarramian A,
Gonzalez Mahave I. In vivo diagnostic tests in adverse reactions to quinolones.
J Investig Allergol Clin Immunol 2007;17:393-8.
116. Araujo L, Demoly P. Macrolides allergy. Curr Pharm Des 2008;14:2840-62.
117. Barni S, Butti D, Mori F, Pucci N, Rossi ME, Cianferoni A, et al. Azi-
thromycin is more allergenic than clarithromycin in children with suspected
hypersensitivity reaction to macrolides. J Investig Allergol Clin Immunol
2015;25:128-32.
118. Benahmed S, Scaramuzza C, Messaad D, Sahla H, Demoly P. The accuracy of
the diagnosis of suspected macrolide antibiotic hypersensitivity: results of a
single-blinded trial. Allergy 2004;59:1130-3.
119. Chia FL, Thong BY. Macrolide allergy: which tests are really useful? Allergol
Immunopathol (Madr) 2011;39:191-2.
120. Lammintausta K, Kortekangas-Savolainen O. Oral challenge in patients with
suspected cutaneous adverse drug reactions: findings in 784 patients during a
25-year-period. Acta Derm Venereol 2005;85:491-6.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S100 BROYLES ET AL
121. Kuyucu S, Mori F, Atanaskovic-Markovic M, Caubet JC, Terreehorst I,
Gomes E, et al. Hypersensitivity reactions to non-betalactam antibiotics in
children: an extensive review. Pediatr Allergy Immunol 2014;25:534-43.
122. Mori F, Pecorari L, Pantano S, Rossi ME, Pucci N, De Martino M, et al.
Azithromycin anaphylaxis in children. Int J Immunopathol Pharmacol 2014;
27:121-6.
123. Cavkaytar O, Karaatmaca B, Yilmaz EA, Sekerel BE, Soyer O. Testing for
clarithromycin hypersensitivity: a diagnostic challenge in childhood. J Allergy
Clin Immunol Pract 2016;4:330-2.e1.
124. Saito R, Sawada Y, Nakamura M. Two cases of eczematous drug eruption caused
by oral tacrolimus administration. Contact Dermatitis 2017;77:128-30.
125. Poveda-Montoyo I, Alvarez-Chinchilla PJ, Garcia Del Pozo MC, Encabo B,
Silvestre JF. Spiramycin-related cutaneous eruption confirmed by patch
testing. Contact Dermatitis 2018;78:233-4.
126. Bianchi L, Caraffini S, Lisi P. Drug reaction with eosinophilia and systemic
symptoms syndrome caused by an everolimus-eluting stent. Int J Dermatol
2014;53:e286-e288.
127. Shin HW, Nam CW, Kim H, Hur SH, Kim YN , Kim KB, et al. Zotarolimus-
eluting stent-induced hypersensitivity pneumonitis. Korean J Intern Med 2013;
28:108-11.
128. Riley L, Mudd L, Baize T, Herzig R. Cross-sensitivity reaction between
tacrolimus and macrolide antibiotics. Bone Marrow Transplant 2000;25:
907-8.
129. Holmes NE, Hodgkinson M, Dendle C, Korman TM. Report of oral clari-
thromycin desensitization. Br J Clin Pharmacol 2008;66:323-4.
130. Nucera E, Roncallo C, Masini L, Buonomo A, De Pasquale T, Pollastrini E,
et al. Successful tolerance induction to spiramycin in pregnancy. Scand J Infect
Dis 2002;34:550-1.
131. Swamy N, Laurie SA, Ruiz-Huidobro E, Khan DA. Successful clarithromycin
desensitization in a multiple macrolide-allergic patient. Ann Allergy Asthma
Immunol 2010;105:489-90.
132. Webster GF, Graber EM. Antibiotic treatment for acne vulgaris. Semin Cutan
Med Surg 2008;27:183-7.
133. Kalai C, Brand R, Yu L. Minocycline-induced Sweet syndrome (acute febrile
neutrophilic dermatosis). J Am Acad Dermatol 2012;67:e289-91.
134. Lebrun-Vignes B, Kreft-Jais C, Castot A, Chosidow O, French Network of
Regional Centers of Pharmacovigilance. Comparative analysis of adverse drug
reactions to tetracyclines: results of a French national survey and review of the
literature. Br J Dermatol 2012;166:1333-41.
135. Maubec E, Wolkenstein P, Loriot MA, Wechsler J, Mulot C, Beaune P, et al.
Minocycline-induced DRESS: evidence for accumulation of the culprit drug.
Dermatology 2008;216:200-4.
136. Talsania N, O’Toole EA. Severe hypersensitivity reaction to minocycline in
association with lymphomatoid papulosis. Clin Exp Dermatol 2009;34:e397-8.
137. Jang JW, Bae YJ, Kim YG, Jin YJ, Park KS, Cho YS, et al. A case of
anaphylaxis to oral minocycline. J Korean Med Sci 2010;25:1231-3.
138. Ogita A, Takada K, Kawana S. Case of anaphylaxis due to tetracycline hy-
drochloride. J Dermatol 2011;38:597-9.
139. Raeder JC. Anaphylactoid reacti on caused by intravenous doxycycline during
general anesthesia and beta-blockade treatment. Drug Intell Clin Pharm 1984;
18:481-2.
140. Shao QQ, Qin L, Ruan GR, Chen RX, Luan ZJ, Ma XJ. Tigecycline-induced
drug fever and leukemoid reaction: a case report. Medicine (Baltimore) 2015;
94:e1869.
141. Correia O, Delgado L, Polonia J. Genital fixed drug eruption: cross-reactivity
between doxycycline and minocycline. Clin Exp Dermatol 1999;24:137.
142. Maciag MC, Ward SL, O’Connell AE, Broyles AD. Hypersensitivity to tet-
racyclines: skin testing, graded challenge, and desensitization regimens. Ann
Allergy Asthma Immunol 2020;124:589-93.
143. Polk RE, Healy DP, Schwartz LB, Rock DT, Garson ML, Roller K. Vanco-
mycin and the red-man syndrome: pharmacodynamics of histamine release.
J Infect Dis 1988;157:502-7.
144. Anne S, Middleton E Jr, Reisman RE. Vancomycin anaphylaxis and successful
desensitization. Ann Allergy 1994;73:402-4.
145. Hwang MJ, Do JY, Choi EW, Seo JH, Nam YJ, Yoon KW, et al. Immuno-
globulin E-mediated hypersensitivity reaction after intraperitoneal adminis-
tration of vancomycin. Kidney Res Clin Pract 2015;34:57-9.
146. Polk RE, Israel D, Wang J, Venitz J, Miller J, Stotka J. Vancomycin skin tests
and prediction of “red man syndrome” in healthy volunteers. Antimicrob
Agents Chemother 1993;37:2139-43.
147. Rwandamuriye FX, Chopra A, Konvinse KC, Choo L, Trubiano JA,
Shaffer
CM, et al. A rapid allele-specific assay for HLA-A*32:01 to identify
patients at risk for vancomycin-induced drug reaction with eosinophilia and
systemic symptoms. J Mol Diagn 2019;21:782-9.
148. Wong JT, Ling M, Patil S, Banerji A, Long A. Oxaliplatin hypersensitivity:
evaluation, implications of skin testing, and desensitization. J Allergy Clin
Immunol Pract 2014;2:40-5.
149. Wong JT, Nagy CS, Krinzman SJ, Maclean JA, Bloch KJ. Rapid oral chal-
lenge-desensitization for patients with aspirin-related urticaria-angioedema.
J Allergy Clin Immunol 2000;105:997-1001.
150. Wong JT, Ripple RE, MacLean JA, Marks DR, Bloch KJ. Vancomycin hy-
persensitivity: synergism with narcotics and “desensitization” by a rapid
continuous intravenous protocol. J Allergy Clin Immunol 1994;94:189-94.
151. Haw W. Vancomycin-specific T cell responses and teicoplanin cross-reac-
tivity. Clin Transl Allergy 2016;164.
152. Blumenthal KG, Patil SU, Long AA. The importance of vancomycin in drug
rash with eosinophilia and systemic symptoms (DRESS) syndrome. Allergy
Asthma Proc 2012;33:165-71.
153. Waldman MA, Black DR, Callen JP. Vancomycin-induced linear IgA bullous
disease presenting as toxic epidermal necrolysis. Clin Exp Dermatol 2004;29:
633-6.
154. Hsiao SH, Chou CH, Lin WL, Lee EJ, Liao LH, Chang HJ, et al. High risk of
cross-reactivity between vancomycin and sequential teicoplanin therapy. J Clin
Pharm Ther 2012;37:296-300.
155. Hung YP, Lee NY, Chang CM, Lee HC, Wu CJ, Chen PL, et al. Tolerability of
teicoplanin in 117 hospitalized adults with previous vancomycin-induced fe-
ver, rash, or neutropenia: a retrospective chart review. Clin Ther 2009;31:
1977-86.
156. Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mech-
anisms of action and resistance and strategies to counter resistance. Antimicrob
Agents Chemother 2000;44:3249-56.
157. Gehrig KA, Warshaw EM. Allergic contact dermatitis to topical antibiotics:
epidemiology, responsible allergens, and management. J Am Acad Dermatol
2008;58:1-21.
158. Bensaid B, Rozieres A, Nosbaum A, Nicolas JF, Berard F. Amikacin-induced
drug reaction with eosinophilia and systemic symptoms syndrome: delayed
skin test and ELISPOT assay results allow the identification of the culprit drug.
J Allergy Clin Immunol 2012;130:1413-4.
159. Hmouda H, Laouani-Kechrid C, Nejib Karoui M, Denguezli M, Nouira R,
Ghannouchi G. A rare case of streptomycin-induced toxic epidermal necrolysis
in a patient with tuber culosis: a therapeutic dilemma. Ann Pharmacother 2005;
39:165-8.
160. Paniagua MJ, Garcia-Ortega P, Tella R, Gaig P, Richart C. Systemic contact
dermatitis to gentamicin. Allergy 2002;57:1086-7.
161. Proebstle TM, Jugert FK, Merk HF, Gall H. Severe anaphylactic reaction to
topical admin istration of framycetin. J Allergy Clin Immunol 1995;96:429-30.
162. Connolly M, McAdoo J, Bourke JF. Gentamicin-induced anaphylaxis. Ir J
Med Sci 2007;176:317-8.
163. Earl HS, Sullivan TJ. Acute desensitization of a patient with cystic fibrosis
allergic to both beta-lactam and aminoglycoside antibiotics. J Allergy Clin
Immunol 1987;79:477-83.
164. Jung DM, Kim JH, Choi NY, Choi JH, Hwang YI, Jang SH, et al. Anaphylaxis
associated with streptomycin skin testing. Ann Allergy Asthma Immunol 2014;
112:81-2.
165. Schulze S, Wollina U. Gentamicin-induced anaphylaxis. Allergy 2003;58:
88-9.
166. Spigarelli MG, Hurwitz ME, Nasr SZ. Hypersensitivity to inhaled TOBI
Ò
following reaction to gentamicin. Pediatr Pulmonol 2002;33:311-4.
167. Legere HJ III, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe
protocol for rapid desensitization in patients with cystic fibrosis and antibiotic
hypersensitivity. J Cyst Fibros 2009;8:418-24.
168. Gurwith MJ, Rabin HR, Love K. Diarrhea associated with clindamycin and
ampicillin therapy: preliminary results of a cooperative study. J Infect Dis
1977;135:S104-10.
169. Bulloch MN, Baccas JT, Arnold S. Clindamycin-induced hypersensitivity re-
action. Infection 2016;44:357-9.
170. Chiou CS, Lin SM, Lin SP, Chang WG, Chan KH, Ting CK. Clindamycin-
induced anaphylactic shock during general anesthesia. J Chin Med Assoc
2006;69:549-51.
171. Lochmann O, Kohout P, Vymola F. Anaphylactic shock following the
administration of clindamycin. J Hyg Epidemiol Microbiol Immunol 1977;21:
441-7.
172. Fass RJ, Scholand JF, Hodges GR, Saslaw S. Clindamycin in the treatment of
serious anaerobic infections. Ann Intern Med 1973;78:853-9.
173. Geddes AM, Bridgwater FA, Williams DN, Oon J, Grimshaw GJ. Clinical and
bacteriological studies with clindamycin. Br Med J 1970;2:703-4.
174. Mazur N, Greenberger PA, Regalado J. Clindamycin hypersensitivity appears
to be rare. Ann Allergy Asthma Immunol 1999;82:443-5.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S101
175. Fulghum DD. Stevens-Johnson syndrome from clindamycin. JAMA 1973;223:
318.
176. Kandula S, Burke WS, Goldfarb JN. Clindamycin-induced Sweet syndrome.
J Am Acad Dermatol 2010;62:898-900.
177. Kapoor R, Flynn C, Heald PW, Kapoor JR. Acute generalized exanthematous
pustulosis induced by clindamycin. Arch Dermatol 2006;142:1080-1.
178. Paquet P, Schaaf-Lafontaine N, Pierard GE. Toxic epidermal necrolysis
following clindamycin treatment. Br J Dermatol 1995;132:665-6.
179. Sahagun Flores JE, Soto Ortiz JA, Tovar Mendez CE, Cardenas Ochoa EC,
Hernandez Flores G. Stevens-Johnson syndrome plus intrahepatic cholestasis
caused by clindamycin or chlorpheniramine. Dermatol Online J 2009;15:12.
180. Schwab RA, Vogel PS, Warschaw KE. Clindamycin-induced acute general-
ized exanthematous pustulosis. Cutis 2000;65:391-3.
181. Sulewski RJ Jr, Blyumin M, Kerdel FA. Acute generalized exanthematous
pustulosis due to clindamycin. Dermatol Online J 2008;14:14.
182. Tian D, Mohan RJ, Stallings G. Drug rash with eosinophilia and
systemic symptoms syndrome associated with clindamycin. Am J Med 2010;
123:e7-8.
183. Valois M, Phillips EJ, Shear NH, Knowles SR. Clindamycin-associated acute
generalized exanthematous pustulosis. Contact Dermat itis 2003;48:169.
184. Morales MP, Carvallo AP, Espinosa KA, Murillo EE. A young man with
myelosuppression caused by clindamycin: a case report. J Med Case Rep 2014;
8:7.
185. Notman MJ, Phillips EJ, Knowles SR, Weber EA, Shear NH. Clindamycin
skin testing has limited diagnostic potential. Contact Dermatitis 2005;53:
335-8.
186. Pereira N, Canelas MM, Santiago F, Brites MM, Goncalo M. Value of patch
tests in clindamycin-related drug eruptions. Contact Dermatitis 2011;65:202-7.
187. Seitz CS, Brocker EB, Trautmann A. Allergy diagnostic testing in clindamy-
cin-induced skin reactions. Int Arch Allergy Immunol 2009;149:246-50.
188. Marcos C, Sopena B, Luna I, Gonzalez R, de la Fuente J, Martinez-Vazquez C.
Clindamycin desensitization in an AIDS patient. AIDS 1995;9:1201-2.
189. Esty B, Minnicozzi S, Chu EC, Broyles AD, Yee CSK. Successful rapid oral
clindamycin desensitization in a pediatric patient. J Allergy Clin Immunol
Pract 2018;6:2141-2.
190. Chen C, Guo DH, Cao X, Cai Y, Xu Y, Zhu M, et al. Risk factors for
thrombocytopenia in adult Chinese patients receiving linezolid therapy. Curr
Ther Res Clin Exp 2012;73:195-206.
191. Hiraki Y, Tsuji Y, Hiraike M, Misumi N, Matsumoto K, Morita K, et al.
Correlation between serum linezolid concentration and the development of
thrombocytopenia. Scand J Infect Dis 2012;44:60-4.
192. Niwa T, Suzuki A, Sakakibara S, Kasahara S, Yasuda M, Fukao A, et al.
Retrospective cohort chart review study of factors associated with the devel-
opment of thrombocytopenia in adult Japanese patients who received intra-
venous linezolid therapy. Clin Ther 2009;31:2126-33.
193. Wu VC, Wang YT, Wang CY, Tsai IJ, Wu KD, Hwang JJ, et al. High fre-
quency of linezolid-associated thrombocytopenia and anemia among patients
with end-stage renal disease. Clin Infect Dis 2006;42:66-72.
194. Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH,
Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive
infections: experience from a compassionate-use program. Clin Infect Dis
2003;36:159-68.
195. Bishop E, Melvani S, Howden BP, Charles PG, Grayson ML. Good clinical
outcomes but high rates of adverse reactions during linezolid therapy for
serious infections: a proposed protocol for monitoring therapy in complex
patients. Antimicrob Agents Chemother 2006;50:1599-602.
196. Natsumoto B, Yokota K, Omata F, Furukawa K. Risk factors for linezolid-
associated thrombocytopenia in adult patients. Infection 2014;42:1007-12.
197. Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, et al. He-
matologic effects of linezolid: summary of clinical experience. Antimicrob
Agents Chemother 2002;46:2723-6.
198. Im JH, Baek JH, Kwon HY, Lee JS. Incidence and risk factors of linezolid-
induced lactic acidosis. Int J Infect Dis 2015;31:47-52.
199. Lee E, Burger S, Shah J, Melton C, Mullen M, Warren F, et al. Linezolid-
associated toxic optic neuropathy: a report of 2 cases. Clin Infect Dis 2003;37:
1389-91.
200. Rho JP, Sia IG, Crum BA, Dekutoski MB, Trousdale RT. Linezolid-associated
peripheral neuropathy. Mayo Clin Proc 2004;79:927-30.
201. Zivkovic SA, Lacomis D. Severe sensory neuropathy associated with long-
term linezolid use. Neurology 2005;64:926-7.
202. Bagwell AD, Stollings JL, White KD, Fadugba OO, Choi JJ. Linezolid
desensitization for a patient with multiple medication hypersensitivity re-
actions. Ann Pharmacother 2013;47:e30.
203. Cawley MJ, Lipka O. Intravenous linezolid administered orally: a novel
desensitization strategy. Pharmacotherapy 2006;26:563-8.
204. Yang M, Xu M. Linezolid-induced angioedema and urticaria in a patient with
renal failure. Braz J Infect Dis 2012;16:606-7.
205. Esposito L, Kamar N, Guilbeau-Frugier C, Mehrenberger M, Modesto A,
Rostaing L. Linezolid-induced interstitial nephritis in a kidney-transplant pa-
tient. Clin Nephrol 2007;68:327-9.
206. Hammer MC, Tomada JRT, Rich M, Bonilla H. Linezolid-induced acute
interstitial nephritis. Infect Dis Clin Pract 2009;17:61-2.
207. Savard
S, Desmeules S, Riopel J, Agharazii M. Linezolid-associated acute
interstitial nephritis and drug rash with eosinophilia and systemic symptoms
(DRESS) syndrome. Am J Kidney Dis 2009;54:e17-e20.
208. Nayak S, Nandwani A, Rastogi A, Gupta V. Acute interstitial nephritis and
drug rash with secondary to linezolid. Indian J Nephrol 2012;22:367-9.
209. McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and im-
plications for resistance development in common uropathogens. J Antimicrob
Chemother 1994;33:23-30.
210. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U,
Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of
controlled trials. J Antimicrob Chemother 2015;70:2456-6 4.
211. Fisk AA. Brief recording: anaphylactoid reaction to nitrofurantoin. N Engl J
Med 1957;256:1054.
212. Jick SS, Jick H, Walker AM, Hunter JR. Hospitalizations for pulmonary re-
actions following nitrofurantoin use. Chest 1989;96:512-5.
213. Khorsandian R, Bremer EM, Nodine JH. Anaphylactic reaction caused by
treatment with nitrofurantoin. JAMA 1963;184:500-2.
214. Tykal P, Wilms H. Anaphylactic shock following oral administration of
nitrofurantoin and demonstration of reagins using the heterologous intracuta-
neous test (Prausnitz-Kustner) [in German]. Deutsch Med Wochenschr 1972;
97:256-7.
215. Sovijarvi AR, Lemola M, Stenius B, Idanpaan-Heikkila J. Nitrofurantoin-
induced acute, subacute and chronic pulmonary reactions. Scand J Respir Dis
1977;58:41-50.
216. Liesching T, O’Brien A. Dyspnea, chest pain, and cough: the lurking
culprit. Nitrofurantoin-induced pulmonary toxicity. Postgrad Med 2002;
112:19-20.
217. D’Arcy PF. Nitrofurantoin. Drug Intell Clin Pharm 1985;19:540-7.
218. Sherigar JM, Fazio R, Zuang M, Arsura E. Autoimmune hepatitis induced by
nitrofurantoin: the importance of the autoantibodies for an early diagnosis of
immune disease. Clin Pract 2012;2:e83.
219. Kelly BD, Heneghan MA, Bennani F, Connolly CE, O’Gorman TA. Nitro-
furantoin-induced hepatotoxicity mediated by CD8þ T cells. Am J Gastro-
enterol 1998;93:819-21.
220. Suntres ZE, Shek PN. Nitrofurantoin-induced pulmonary toxicity: in vivo
evidence for oxidative stress-mediated mechanisms. Biochem Pharmacol 1992;
43:1127-35.
221. Lammintausta K, Kortekangas-Savolainen O. The usefulness of skin tests to
prove drug hypersensitivity. Br J Dermatol 2005;152:968-74.
222. Connell DW, Berry M, Cooke G, Kon OM. Update on tuberculosis: TB in the
early 21st century. Eur Respir Rev 2011;20:71-84.
223. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement.
Global burden of tuberculosis: estimated incidence, prevalence, and mortality
by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;
282:677-86.
224. Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. LTBI:
latent tuberculosis infection or lasting immune responses to M. tuberculosis?A
TBNET consensus statement. Eur Respir J 2009;33:956-73.
225. Aouam K , Chaabane A, Lous saief C, Ben Romdh ane F, Boughattas NA,
Chakroun M. Adverse effec ts of antitubercula r drugs: epidemiolog y,
mechanisms, and patient ma nagement [i n French]. Med Mal I nfect 2007;
37:253-61.
226. Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course
Chemotherapy Trial 21: effectiveness, toxicity, and acceptability. The report of
final results. Ann Intern Med 1990;112:397-406.
227. Nikolaeva OD. Side effects of chemotherapy in patients with pulmonary
tuberculosis and concomitant diseases [in Russian]. Lik Sprava 2003;(3-4):
74-8.
228. Kishore PV, Palaian S, Ojha P, Shankar PR. Pattern of adverse drug reactions
experienced by tuberculosis patients in a tertiary care teaching hospital in
Western Nepal. Pak J Pharm Sci 2008;21:51-6.
229. Snider DE Jr, Long MW, Cross FS, Farer LS. Six-months isoniazid-rifampin
therapy for pulmonary tuberculosis: report of a United States Public Health
Service Cooperative Trial. Am Rev Respir Dis 1984;129:573-9.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S102 BROYLES ET AL
230. Vieira DE, Gomes M. Adverse effects of tuberculosis treatment: experience at
an outpatient clinic of a teaching hospital in the city of Sao Paulo, Brazil.
J Bras Pneumol 2008;34:1049-55.
231. Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S,
Haahtela T, et al. A revised nomenclature for allergy. An EAACI position
statement from the EAACI Nomenclature Task Force. Allergy 2001;56:
813-24.
232. Aristoff PA, Garcia GA, Kirchhoff PD, Showalter HD. Rifamycins–obstacles
and opportunities. Tuberculosis (Edinb) 2010;90:94-118.
233. Martinez E, Collazos J, Mayo J. Hypersensitivity reactions to rifampin:
pathogenetic mechanisms, clinical manifestations, management strategies, and
review of the anaphylactic-like reactions. Medicine (Baltimore) 1999;78:
361-9.
234. Broz P, Harr T, Hecking C, Grize L, Scherer K, Jaeger KA, et al. Nonirritant
intradermal skin test concentrations of ciprofloxacin, clarithromycin, and
rifampicin. Allergy 2012;67:647-52.
235. Buergin S, Scherer K, Hausermann P, Bircher AJ. Immediate hypersensitivity
to rifampicin in 3 patients: diagnostic procedures and induction of clinical
tolerance. Int Arch Allergy Immunol 2006;140:20-6.
236. Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and
pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001;
40:327-41.
237. Matz J, Borish LC, Routes JM, Rosenwasser LJ. Oral desensitization to
rifampin and ethambutol in mycobacterial disease. Am J Respir Crit Care Med
1994;149:815-7.
238. Hildebrand KJ, Atkinson A, Kitai I. Rifampin hypersensitivity in a 2-year-old
child with successful rapid oral desensitization. Pediatr Infect Dis J 2014;33:
787.
239. Kim JH, Kim HB, Kim BS, Hong SJ. Rapid oral desensitization to isoniazid,
rifampin, and ethambutol. Allergy 2003;58:540-1.
240. Logsdon S, Ramirez-Avila L, Castells M, Dioun A. Successful rifampin
desensitization in a pediatric patient with latent tuberculosis. Pediatr Allergy
Immunol 2014;25:404-5.
241. Syrigou E, Grapsa D, Nanou E, Zande M, Vassias A, Gkiozos I, et al.
Anaphylaxis during rapid oral desensitization to rifampicin. J Allergy Clin
Immunol Pract 2016;4:173-4.
242. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC,
Friedman LN, et al. American Thoracic Society/Centers for Disease Control
and Prevention/Infectious Diseases Society of America: treatment of tuber-
culosis. Am J Respir Crit Care Med 2003;167:603-62.
243. Olivier C, Radal M, Mazaud S, Jonville-Bera AP, Martel C, Autret E. Éruption
après une première prise d’une quadrithérapie antituberculeuse: penser au
pyrazinamide [in French]. Arch Pediatr 1998;5:289-90.
244. Radal M, Jonville-Bera AP, Van-Egroo C, Carre P, Lemarie E, Autret E.
Eruption after the 1st dose of standard antitubercular chemotherapy: thoughts
on pyrazinamide. Rev Mal Respir 1998;15:305-6.
245. Vervloet D, Pradal M, Castela M. Drug Allergy. Uppsala, Sweden: Pharmacia
& UpJohn; 1999.
246. Bavbek S, Yilmaz I, Aydin O, Ozdemir SK. Pyrazinamide-induced anaphy-
laxis: diagnosed by skin test and successful desensitization. Int Arch Allergy
Immunol 2012;157:209-12.
247. Holdiness MR. Adver se cutaneous reactions to antituberculosis drugs. Int J
Dermatol 1985;24:280-5.
248. Durand F, Pessayre D, Fournier M, Belghiti J, Erlinger S, Bernuau J. Anti-
tuberculous therapy and acute liver failure. Lancet 1995;345:1170.
249. Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Derma-
titis 1986;15:282-8.
250. Nariman S. Adverse reactions to drugs used in the treatment of tuberculosis.
Adverse Drug React Acute Poisoning Rev 1988;7:207-27.
251. Rubira N, Baltasar MA, Marti E. Hypersensitivity syndrom e from isoniazid.
Allergy 1999;54:1011-2.
252. Herrejon Silvestre A, Furest Carrasco I, Marin Gonzalez M. Fiebre por iso-
niacida. Arch Bronconeumol 2000;36:112-3.
253. Lee CH, Hsiue TR, Chen CW, Chang HY, Chen CR. Isoniazid-induced fever.
J Formos Med Assoc 1996;95:632-4.
254. Bakkum RS, Waard-Van Der Spek FB, Thio HB. Delayed-type hypersensi-
tivity reaction to ethambutol and isoniazid. Contact Dermatitis 2002;46:359.
255. Girling DJ. Adverse effects of antituber culosis drugs. Drugs 1982;23:56-74.
256. Honeycutt WM. Reactions to isoniazid. Arch Dermatol 1963;88:190.
257. Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA,
Snodgrass WR, et al. Isoniazid liver injury: clinical spectrum, pathology, and
probable pathogenesis. Ann Intern Med 1976;84:181-92.
258. Lafourcade MP, Martinl M, Revolte X, Ba L, Hamoudi M, Bara R. Reaction
allergique aux antituberculeux majeurs. Revue Francaise d’Allergologie 2009;
49:496-9.
259. Rodrigues Carvalho S, Silva I, Leiria-Pinto P, Rosado-Pinto J. Rapid oral
tolerance induction to isoniazid and pyrazinamide and controlled administra-
tion
of ethambutol: clinical case. Allergol Immunopathol (Madr) 2009;37:
336-8.
260. Abadoglu O, Epozturk K, Atayik E. Rapid oral desensitisation to prophylactic
isoniazid. Allergol Immunopathol (Madr) 2011;39:311-2.
261. Rodrigues J, Moreira A, Fonseca J, Vaz M. Oral desensitization to izoniazid.
R Port Alergologia 2007;9:263-4.
262. Griffith DE, Brown-Elliott BA, Shepherd S, McLarty J, Griffith L, Wallace RJ Jr.
Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium
complex lung disease. Am J Respir Crit Care Med 2005;172:250-3 .
263. Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP. High hepato-
toxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis.
Eur Respir J 2005;26:462-4.
264. Brunton LL, Lazo JS, Parker KL. Goodman & Gilman’s the Pharmacological
Basis of Therapeutics. 11th ed. New York: McGraw-Hill Professional; 2006.
265. Grossman ME, Warren K, Mady A, Satra KH. Lichenoid eruption associated
with ethambutol. J Am Acad Dermatol 1995;33:675-6.
266. HiraokaK,NagataN,SuzukiK,Kawajiri T, Kurokawa S, Kawamura T,
et al. A case of pulmon ary reaction with skin eruption showing a positive
peripheral lymp hocyte stim ulation tes t result for etham butol. J UOEH
1998;20:145-51.
267. Pegram PS Jr, Mountz JD, O’Bar PR. Ethambutol-induced toxic epidermal
necrolysis. Arch Intern Med 1981;141:1677-8.
268. Srivastava N, Solanki LS, Chand S, Garbyal RS, Singh S. Ashy dermatosis-
like pigmentation due to ethambutol. Indian J Dermatol Venereol Leprol 2008;
74:281-2.
269. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General
considerations for skin test procedures in the diagnosis of drug hypersensi-
tivity. Allergy 2002;57:45-51.
270. Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al.
General considerations on rapid desensitization for drug hypersensitivity—a
consensus statement. Allergy 2010;65:1357-66.
271. Cernadas JR, Santos N, Pinto C, Mota PC, Castells M. Hypersensitivity re-
action and tolerance induction to ethambutol. Allergy 2011;66:381.
272. Castells MC, Tennant NM, Sloane DE, Ida Hsu F, Barrett NA , Hong DI, et al.
Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid
desensitization in 413 cases. J Allergy Clin Immunol 2008;122:574-80.
273. Athira B, Manju CS, Jyothi E. A study on adverse drug reactions to first line
antitubercular drugs in DOTS therapy. Int J Pharmacol Clin Sci 2015;4:7-11.
274. Macpherson P. Sensitization to P.A.S., streptomycin, and isoniazid. Br Med J
1957;2:505-6.
275. Sanchez-Borges M, Thong B, Blanca M, Ensina LF, Gonzalez-Diaz S,
Greenberger PA, et al. Hypersensitivity reactions to non beta-lactam antimi-
crobial agents, a statement of the WAO special committee on drug allergy.
World Allergy Organ J 2013;6:18.
276. Chakravarty S. A method of desensitization of allergy due to streptomycin with
prednisone. Dis Chest 1957;32:310-4.
277. Russell B. Desensitization to streptomycin and P.A.S. Br Med J 1953;2:1322.
278. Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for
treatment of anaerobic infections. Clin Infect Dis 2010;50:S16-23.
279. Workowski KA, Bolan GA, Centers for Disease Control and Prevention.
Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm
Rep 2015;64:1-137.
280. Asensio Sanchez T, Davila I, Moreno E, Laffond E, Macias E, Ruiz A, et al.
Anaphylaxis due to metronidazole with positive skin prick test. J Investig
Allergol Clin Immunol 2008;18:138-9.
281. Gastaminza G, Anda M, Audicana MT, Fernandez E, Munoz D. Fixed-drug
eruption due to metronidazole with positive topical provocation. Contact
Dermatitis 2001;44:36.
282. Kumar N, Sundriyal D, Walia M, Trisal D. Metronidazole-induced fixed drug
eruption. BMJ Case Rep 2013;2013:bcr2013200470.
283. Mazumdar G, Shome K. Stevens-Johnson syndrome following use of metro-
nidazole in a dental patient. Indian J Pharmacol 2014;46:121-2.
284. Short KA, Fuller LC, Salisbury JR. Fixed drug eruption following metroni-
dazole therapy and the use of topical provocation testing in diagnosis. Clin Exp
Dermatol 2002;27:464-6.
285. Weart CW, Hyman LC. Serum sickness associated with metronidazole.
Southern Med J 1983;76:410-1.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S103
286. Garcia-Rubio I, Martinez-Cocera C, Santos Magadan S, Rodriguez-Jimenez B,
Vazquez-Cortes S. Hypersensitivity reactions to metronidazole. Allergol
Immunopathol (Madr) 2006;34:70-2.
287. Gendelman SR, Pien LC, Gutta RC, Abouhassan SR. Modified oral metroni-
dazole desensitization protocol. Allergy Rhinol (Providence) 2014;5:66-9.
288. Kurohara ML, Kwong FK, Lebherz TB, Klaustermeyer WB. Metronidazole
hypersensitivity and oral desensitization. J Allergy Clin Immunol 1991;88:
279-80.
289. Pearlman MD, Yashar C, Ernst S, Solomon W. An incremental dosing pro-
tocol for women with severe vaginal trichomoniasis and adverse reaction to
metronidazole. Am J Obstet Gynecol 1996;174:934-6.
290. Helms DJ, Mosure DJ, Secor WE, Workowski KA. Management of tricho-
monas vaginalis in women with suspected metronidazole hypersensitivity. Am
J Obstet Gynecol 2008;198:370.e1-7.
291. Kanwar AJ, Sharma R, Rajagopalan M, Kaur S. Fixed drug eruption due to
tinidazole with cross-reactivity with metronidazole. Dermatologica 1990;180:
277.
292. Mishra D, Mobashir M, Zaheer MS. Fixed drug eruption and cross-reactivity
between tinidazole and metronidazole. Int J Dermatol 1990;29:740.
293. Thami GP, Kanwar AJ. Fixed drug eruption due to metronidazole and tini-
dazole without cross-sensitivity to secnidazole. Dermatology 1998;196:368.
294. Petersen E, Ronne T, Ronn A, Bygbjerg I, Larsen SO. Reported side effects to
chloroquine, chloroquine plus proguanil, and mefloquine as chemoprophylaxis
against malaria in Danish travelers. J Travel Med 2000;7:79-84.
295. Ekpechi OL, Okoro AN. A pattern of pruritus due to chloroquine. Arch
Dermatol 1964;89:631-2.
296. Boffa MJ, Chalmers RJ. Toxic epidermal necrolysis due to chloroquine
phosphate. Br J Dermatol 1994;131:444-5.
297. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:
25-61.
298. Smith HR, Croft AM, Black MM. Dermatological adverse effects with the
antimalarial drug mefloquine: a review of 74 published case reports. Clin Exp
Dermatol 1999;24:249-54.
299. Deen JL, von Seidlein L, Dondorp A. Therapy of uncomplicated malaria in
children: a review of treatment principles, essential drugs and current recom-
mendations. Trop Med Int Health 2008;13:1111-30.
300. Looareesuwan S, Chulay JD, Canfield CJ, Hutchinson DB. Malarone (atova-
quone and proguanil hydrochloride): a review of its clinical development for
treatment of malaria. Malarone Clinical Trials Study Group. Am J Trop Med
Hyg 1999;60:533-41.
301. McKeage K, Scott L. Atovaquone/proguanil: a review of its use for the pro-
phylaxis of Plasmodium falciparum malaria. Drugs 2003;63:597-623.
302. Emberger M, Lechner AM, Zelger B. Stevens-Johnson syndrome associated
with Malarone antimalarial prophylaxis. Clin Infect Dis 2003;37:e5-7.
303. Just N, Carpentier O, Brzezinki C, Steenhouwer F, Staumont-Salle D. Severe
hypersensitivity reaction as acute eosinophil ic pneumonia and skin eruption
induced by proguanil. Eur Respir J 2011;37:1526-8 .
304. Kremsner PG, Looareesuwan S, Chulay JD. Atovaquone and proguanil hy-
drochloride for treatment of malaria. J Travel Med 1999;6:S18-20.
305. Janier M, Froidevaux D, Lons-Danic D, Daniel F. Acute generalized exan-
thematous pustulosis due to the combination of chloroquine and proguanil.
Dermatology 1998;196:271.
306. Luong MS, Bessis D, Raison-Peyron N, Pinzani V, Guilhou JJ, Guillot B.
Severe mucocutaneous necrotizing vasculitis associated with the combination
of chlor oquine and proguanil. Acta Derm Venereol 2003;83:141.
307. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and
the dapsone hypersensitivity syndrome. N Engl J Med 2013;369:1620-8.
308. Price R, van Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R,
et al. Adverse effects in patients with acute falciparum malaria treated with
artemisinin derivatives. Am J Trop Med Hyg 1999;60:547-55.
309. Leonardi E, Gilvary G, White NJ, Nosten F. Severe allergic reactions to oral
artesunate: a report of two cases. Trans R Soc Trop Med Hyg 2001;95:182-3.
310. AlKadi HO. Antimalarial drug toxicity: a review. Chemotherapy 2007;53:
385-91.
311. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis 2006;
19:588-93.
312. Ottesen EA, Campbell WC. Ivermectin in human medicine. J Antimicrob
Chemother 1994;34:195-203.
313. Fujimoto K, Kawasaki Y, Morimoto K, Kikuchi I, Kawana S. Treatment for
crusted scabies: limitations and side effects of treatment with ivermectin.
J Nippon Med Sch 2014;81:157-63.
314. Gann PH, Neva FA, Gam AA. A randomized trial of single- and two-do se
ivermectin
versus thiabendazole for treatment of strongyloidiasis. J Infect Dis
1994;169:1076-9.
315. Orion E, Matz H, Wolf R. The life-threatening complications of dermatologic
therapies. Clin Dermatol 2005;23:182-92.
316. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans.
Parasitology 2000;121:S113-S132.
317. Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM . A meta-
analysis of the effectiveness of albendazole compared with metronidazole as
treatments for infections with Giardia duodena lis . PLoS Negl Trop Dis 2010;
4:e682.
318. Macedo NA, Pineyro MI, Carmona C. Contact urticaria and contact dermatitis
from albendazole. Contact Dermatitis 1991;25:73-5.
319. Mahboob A, Haroon TS. Fixed drug eruption with albendazole and its cross-
sensitivity with metronidazole–a case report. J Pak Med Assoc 1998;4 8:316-7.
320. Bagheri H, Simiand E, Montastruc JL, Magnaval JF. Adverse drug reactions to
anthelmintics. Ann Pharmacother 2004;38:383-8.
321. Chen KT, Twu SJ, Chang HJ, Lin RS. Outbreak of Stevens-Johnson syn-
drome/toxic epidermal necrolysis associated with mebendazole and metroni-
dazole use among Filipino laborers in Taiwan. Am J Public Health 2003;93:
489-92.
322. Grove DI. Treatment of strongyloidiasis with thiabendazole: an analysis of
toxicity and effectiveness. Trans R Soc Trop Med Hyg 1982;76:114-8.
323. Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for in-
testinal and urinary schistosomiasis—a met a-analysis of comparative and non-
comparative clinical trials. PLoS Negl Trop Dis 2014;8:e3286.
324. Huang SW. A clinical approach to a patient with praziquantel hypersensitivity.
J Allergy Clin Immunol 1992;90:867.
325. Kyung SY, Cho YK, Kim YJ, Park JW, Jeong SH, Lee JI, et al.
A paragonimiasis patient with allergic reaction to praziquantel and resistance to
triclabendazole: successful treatment after desensitization to praziquantel.
Korean J Parasitol 2011;49:73-7.
326. Lee JM, Lim HS, Hong ST. Hypersensitive reaction to praziquantel in a clo-
norchiasis patient. Korean J Parasitol 2011;49:273-5.
327. Shen C, Choi MH, Bae YM, Yu G, Wang S, Hong ST. A case of anaphylactic
reaction to praziquantel treatment. Am J Trop Med Hyg 2007;76:603-5.
328. Matsumoto J. Adverse effects of praziquantel treatment of Schistosoma
japonicum infection: involvement of host anaphylactic reactions induced by
parasite antigen release. Int J Parasitol 2002;32:461-71.
329. Francis H, Awadzi K, Ottesen EA. The Mazzotti reaction following treatment
of onchocerciasis with diethylcarbamazine: clinical severity as a function of
infection intensity. Am J Trop Med Hyg 1985;34:529-36.
330. Docampo R, Moreno SN. Current chem otherapy of human African trypano-
somiasis. Parasitol Res 2003;90:S10-3.
331. Blum J, Nkunku S, Burri C. Clinical description of encephalopathic syn-
dromes and risk factors for their occurrence and outcome during melarso-
prol treatment of human African trypanosomiasis. Trop Med Int Health
2001;6:390-400.
332. Gonzalez-Mendiola R, Martinez Borque N, Palomeque Rodriguez T, Torre-
cillas Toro M, Martinez Bohigas D. Type I allergic reaction to benzimidazole
antihelmintics. Allergy 2007;62:713-4.
333. Nett JE, Andes DR. Antifungal agents: spectrum of activity, pharmacology,
and clinical indications. Infect Dis Clin North Am 2016;30:51-83.
334. Zonios DI, Bennett JE. Update on azole antifungals. Semin Respir Crit Care
Med 2008;29:198-210.
335. Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of anti-
fungals. Ann Pharmacother 2009;43:1647-57.
336. Bittleman DB, Stapleton J, Casale TB. Report of successful desensitization to
itraconazole. J Allergy Clin Immunol 1994;94:270-1.
337. Craig TJ, Peralta F, Boggavarapu J. Desensitization for fluconazole hyper-
sensitivity. J Allergy Clin Immunol 1996;98:845-6.
338. Jariwala S, Vernon N, de Vos G. A novel method of desensitization for flu-
conazole hypersensitivity in a patient with AIDS. Ann Allergy Asthma
Immunol 2011;106:542-3.
339. Jean T, Kwong K. Successful desensitization of voriconazole in an immuno-
suppressed pediatric patient. J Allergy Clin Immunol Pract 2015;3:637-8.
340. Arrieta
AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical
trials. Pediatr Infect Dis J 2011;30:e97-e102.
341. Zaoutis TE, Jafri HS, Huang LM, Locatelli F, Barzilai A, Ebell W, et al.
A prospective, multicenter study of caspofungin for the treatment of docu-
mented Candida or Aspergillus infections in pediatric patients. Pediatrics 2009;
123:877-84.
342. Lee MC, Ni YW, Wang CH, Lee CH, Wu TW. Caspofungin-induced severe
toxic epidermal necrolysis. Ann Pharmacother 2010;44:1116-8.
343. Patel S, Alangaden GJ, Lum LG, Cronin SM, Abidi MH, Dieterle N, et al.
Immediate cross-hypersensitivity between micafungin and caspofungin: a case
report. J Oncol Pharm Pract 2009;15:187-9.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S104 BROYLES ET AL
344. Randolph C, Kaplan C, Fraser B. Rapid desensitization to fluconazole
(Diflucan). Ann Allergy Asthma Immunol 2008;100:616-7.
345. Ward SL, Maciag MC, Jones S, Lee J, Lee J, Broyles A. Successful rapid
desensitization to micafungin in a pediatric patient [published online ahead of
print July 21, 2020]. Ped Allergy Immunol Pulmonmol. https://doi.org/10.
1089/ped.2020.1204.
346. Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al.
Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl
J Med 2015;373:795-807.
347. EMPRANO ANRS 12136 Study GroupDanel C, Moh R, Gabillard D,
Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid pre-
ventive therapy in Africa. N Engl J Med 2015;373:808-22.
348. Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al.
Antiretroviral drugs for treatment and prevention of HIV infection in Adults:
2016 Recommendations of the International Antiviral Society-USA Panel.
JAMA 2016;316:191-210.
349. Milpied-Homsi B, Moran EM, Phillips EJ. Antiviral drug allergy. Immunol
Allergy Clin North Am 2014;34:645-62.
350. Putterman C, Rahav G, Shalit M, Rubinow A. “Treating through” hypersen-
sitivity to co-trimoxazole in AIDS patients. Lancet 1990;336:52.
351. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al.
HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;
358:568-79.
352. Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, et al. High
sensitivity of human leukocyte antigen-b*5701 as a marker for immunologi-
cally confirmed abacavir hypersensitivity in white and black patients. Clin
Infect Dis 2008;46:1111-8.
353. White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the
immunopathogenesis of T cell-mediated drug allergy: the role of host, path-
ogens, and drug response. J Allergy Clin Immunol 2015;136:219-34.
354. Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with
abacavir. AIDS 1999;13:999-1000.
355. Strazzula L, Pratt DS, Zardas J, Chung RT, Thiim M, Kroshinsky D. Wide-
spread morbilliform eruption associated with telaprevir: use of dermatologic
consultation to increase tolerability. JAMA Dermatol 2014;150:756-9.
356. Eyre ZW, Secrest AM, Woodcock JL. Photo-induced drug eruption in a patient
on combination simeprevir/sofosbuvir for hepatitis C. JAAD Case Rep 2016;2:
224-6.
357. Simpson CL, McCausland D, Chu EY. Photo-distributed lichenoid eruption
secondary to direct anti-viral therapy for hepatitis C. J Cutan Pathol 2015;42:
769-73.
358. Ebo DG, Bridts CH, De Clerck LS, Stevens WJ. Immediate allergy from
valacyclovir. Allergy 2008;63:941-2.
359. Lammintausta K, Makela L, Kalimo K. Rapid systemic valaciclovir reaction
subsequent to aciclovir contact allergy. Contact Dermatitis 2001;45:181.
360. Shah SA, Gulbis A, Wilhelm K. A case series using famciclovir in stem cell
transplant recipients with valacyclovir hypersensitivity reactions. J Oncol
Pharm Pract 2015;21:305-9.
361. Hirschfeld G, Weber L, Renkl A, Scharffetter-Kochanek K, Weiss JM.
Anaphylaxis after Oseltamivir (Tamiflu) therapy in a patient with sensitization to
star anise and celery-carrot-mugwort-spice syndrome. Allergy 2008;63:243-4.
362. DeSimone JA, Ojha A, Pathak R, Cohn J. Successful desensitization to
enfuvirtide after a hypersensitivity reaction in an HIV-1-infected man. Clin
Infect Dis 2004;39:e110-2.
363. Marcos Bravo MC, Ocampo Hermida A, Martinez Vilela J, Perez
Rodriguez MT, Gavilan Montenegro MJ, Arenas Villarroel LJ, et al. Hyper-
sensitivity reaction to darunavir and desensitization protocol. J Investig
Allergol Clin Immunol 2009;19:250-1.
364. Quiros-Roldan E, Tirelli V, Torti C, Sosta E, Tosoni C, Damiolini E, et al.
Successful long-course after failure of short-course desensitization in a patient
with severe hypersensitivity reaction to enfuvirtide. AIDS 2007;21:1388-9.
365. Toker O, Tal Y, Daher S, Shalit M. Ribavirin desensitization in chronic
hepatitis C. Isr Med Assoc J 2015;17:583-4.
366. Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, et al.
Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol
1999;17:1141.
367. Polyzos A, Tsavaris N, Kosmas C, Arnaouti T, Kalahanis N, Tsigris C, et al.
Hypersensitivity reactions to carboplatin administration are common but not
always severe: a 10-year experience. Oncology 2001;61:129-33.
368. Hesterberg PE, Banerji A, Oren E, Penson RT, Krasner CN, Seiden MV, et al.
Risk strati fi cation for desensitization of patients with carboplatin hypersensi-
tivity: clinical presentation and management. J Allergy Clin Immunol 2009;
123:1262-7.e1.
369. Patil
SU, Long AA, Ling M, Wilson MT, Hesterberg P, Wong JT, et al.
A protocol for risk stratification of patients with carboplatin-induced hyper-
sensitivity reactions. J Allergy Clin Immunol 2012;129:443-7.
370. Lax T, Long A, Banerji A. Skin testing in the evaluation and management of
carboplatin-related hypersensitivity reactions. J Allergy Clin Immunol Pract
2015;3:856-62.
371. Caiado J, Castells M. Presentation and diagnosis of hypersensitivity to plat-
inum drugs. Curr Allergy Asthma Rep 2015;15:15.
372. Leguy-Seguin V, Jolimoy G, Coudert B, Pernot C, Dalac S, Vabres P, et al.
Diagnostic and predictive value of skin testing in platinum salt hypersensi-
tivity. J Allergy Clin Immunol 2007;1 19:726-30.
373. Pagani M, Bonadonna P, Senna GE, Antico A. Standardization of skin tests for
diagnosis and prevention of hypersensitivity reactions to oxaliplatin. Int Arch
Allergy Immunol 2008;145:54-7.
374. Wang AL, Patil SU, Long AA, Banerji A. Risk-stratification protocol for
carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug
allergy. Ann Allergy Asthma Immunol 2015;115:422-8.
375. Tonini G, Santini D, Vincenzi B, Borzomati D, Dicuonzo G, La Cesa A, et al.
Oxaliplatin may induce cytokine-release syndrome in colorectal cancer pa-
tients. J Biol Regul Homeost Agents 2002;16:105-9.
376. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Man-
agement of hypersensitivity reactions to carboplatin and paclitaxel in an
outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol
Pract 2014;2:428-33.
377. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a
comprehensive review. Clin Rev Allergy Immunol 2015;49:177-91.
378. Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvao VR, Berlin ST, et al.
Risk stratification and skin testing to guide re-exposure in taxane-induced
hypersensitivity reactions. J Allergy Clin Immunol 2016;137:1154-64.e12.
379. Prieto García A, Pineda de la Losa F. Immunoglobulin E-mediated severe
anaphylaxis to paclitaxel. J Investig Allergol Clin Immunol 2010;20:170-1.
380. Alvarez-Cuesta E, Madrigal-Burgaleta R, Angel-Pereira D, Urena-Tavera A,
Zamora-Verduga M, Lopez-Gonzalez P, et al. Delving into cornerstones of
hypersensitivity to antineoplastic and biological agents: value of diagnostic
tools prior to desensitization. Allergy 2015;70:784-94.
381. Otani IM, Lax T, Long AA, Slawski BR, Camargo CA Jr, Banerji A. Utility of
risk stratification for paclita xel hypersensitivity reactions. J Allergy Clin
Immunol Pract 2018;6:1266-1273.e2.
382. Pfizer Laboratories (Pty) Ltd. Methotrexate prescribing information. Available
from: http://labeling.pfizer.c om/ShowLabeling.aspx?id¼1093. Accessed July
28, 2020.
383. Alarcon GS, Kremer JM, Macaluso M, Weinblatt ME, Cannon GW,
Palmer WR, et al. Risk factors for methotrexate-induced lung injury in patients
with rheumatoid arthritis: a multicenter, case-control study. Methotrexate-Lung
Study Group. Ann Intern Med 1997;127:356-64.
384. Caldeira T, Costa V, Silva I, Oliva T, Norton L. Anaphylactoid reaction to
high-dose methotrexate and re-administration after a successful desensitization.
Pediatr Hematol Oncol 2008;25:131-4.
385. Pichler WJ. Drug Hypersensitivity. Bern, Switzerland: Karger AG; 2007.
386. Dilley MA, Lee JP, Broyles AD. Methotrexate hypersensitivity reactions in
pediatrics: evaluation and management. Pediatr Blood Cancer 2017;64.
387. Ruggiero A, Triarico S, Trombatore G, Battista A, Dell’acqua F, Rizzari C,
et al. Incidence, clinical features and management of hypersensitivity reactions
to chemotherapeutic drugs in children with cancer. Eur J Clin Pharmacol 2013;
69:1739-46.
388. Pugi A, Benemei S, Vietri M, Tondo A, Calvani AM, Mugelli A, et al.
Anaphylaxis during the first course of high-dose methotrexate: a case report
and literature review. J Clin Pharm Ther 2012;37:245-8.
389. Davis KA, Williams P, Walker JC. Successful desensitization to high-dose
methotrexate after systemic anaphylaxis. Ann Allergy Asthma Immunol 2003;
90:87-9.
390. Lluch-Bernal M, Cuesta-Herranz J, De las Heras M, Figueredo E,
Umpierrez A, Fernandez M, et al. Anaphylactic reaction to methotrexate.
Allergy 1997;52:1150-1.
391. Vega A, Cabanas R, Contreras J, Lopez Cazana J, Lopez Serrano C, Pascual C,
et al. Anaphylaxis to met hotrexate: a possible IgE-mediated mechanism.
J Allergy Clin Immunol 1994;94:268-70.
392. Bouchireb
K, Dodille A, Ponvert C, Gouraud F, Dubrel M, Brugieres L.
Management and successful desensitization in methotrexate-induced anaphy-
laxis. Pediatr Blood Cancer 2009;52:295-7.
393. Oulego-Erroz I, Maneiro-Freire M, Bouzon-Alejandro M, Vazquez-
Donsion M, Couselo JM. Anaphylactoid reaction to high-dose methotrexate
and successful desensitization. Pediatr Blood Cancer 2010;55:557-9.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S105
394. Scott JR, Ward DA, Crews KR, Panett a JC, Navid F. Hypersensitivity reaction
to high-dose methotrexate and successful rechallenge in a pediatric patient with
osteosarcoma. Pediatr Blood Cancer 2014;61:373-5.
395. Clowse MB, McCune WJ. General toxicity of cyclophosphamide in rheumatic
diseases. UpToDate. 2020. Available from: https://www.uptodate.com/
contents/general-toxicity-of-cyclophosphamide-in-rheumatic-diseases. Accessed
July 28, 2020.
396. Lienesch DW, Mutasim DF, Singh RR. Neutrophilic eccrine hidradenitis
mimicking cutaneous vasculitis in a lupus patient: a complication of cyclo-
phosphamide. Lupus 2003;12:707-9.
397. Kovalyshyn I, Bijal AD, Lacouture ME, Brownell I. Cyclophosphamide-
associated acneiform drug eruption in a patient with multiple myeloma. J Am
Acad Dermatol 2011;65:657-9.
398. Visitsunthorn N, Utsawapreechawong W, Pacharn P, Jirapongsananuruk O,
Vichyanond P. Immediate type hypersensitivity to chemotherapeutic agents in
pediatric patients. Asian Pacific J Allergy Immunol 2009;27:191-7.
399. Rosas-Vargas MA, Casas-Becerra B, Velazquez-Armenta Y, Sienra-Monge JJ,
Del Rio-Navarro BE. Cyclophosphamide hypersensitivity in a leukemic child.
Ther Drug Monit 2005;27:263-4.
400. Chanan-Khan A, Szebeni J, Savay S, Lieb es L, Rafique NM, Alving CR, et al.
Complement activation following first exposure to pegylated liposomal
doxorubicin (Doxil ): possible role in hypersensitivity reactions. Ann Oncol
2003;14:1430-7.
401. Joly F, Ray-Coquard I, Fabbro M, Donoghoe M, Boman K, Sugimoto A, et al.
Decreased hypersensitivity reactions with carboplatin-pegylated liposomal doxo-
rubicin compared to carboplatin-paclitaxel combination: analysis from the GCIG
CALYPSO relapsing ovarian cancer trial. Gynecol Oncol 2011;122:226-32.
402. Farr KP, Safwat A. Palmar-plantar erythrodysesthesia associated with
chemotherapy and its treatment. Case Rep Oncol 2011;4:229-35.
403. Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs
and targeted therapies for cancer, part I: conventional chemotherapeutic drugs.
J Am Acad Dermatol 2014;71:203.e1-2.
404. Balsari A, Lombardo N, Ghione M. Skin and perivascular toxicity induced
experimentally by doxorubicin. J Chemother 1989;1:324-9.
405. Lopes G, Vincek V, Raez LE. Pemetrexed-associated urticarial vasculitis.
Lung Cancer 2006;51:247-9.
406. Phillips J, Kujawa J, Davis-Lorton M, Hindenburg A. Successful desensiti-
zation in a patient with lenalidomide hypersensitivity. Am J Hematol 2007;82:
1030.
407. Hall VC, El-Azhary RA, Bouwhuis S, Rajkumar SV. Dermatologic side effects
of thalidomide in patients with multiple myeloma. J Am Acad Dermatol 2003;
48:548-52.
408. Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering
thalidomide: a review of its mechanism of action, side effects, and potential
uses. J Am Acad Dermatol 1996;35:969-79.
409. Celgene Corporation. THALOMID REMSÒ. 2017. Available from: https://
www.thalomidrems.com/. Accessed July 28, 2020.
410. Barley K, He W, Agarwal S, Jagannath S, Chari A. Outcomes and manage-
ment of lenalidomide-associated rash in patients with multiple myeloma. Leuk
Lymphoma 2016;57:2510-5.
411. Rajkumar SV, Gertz MA, Witzig TE. Life-threatening toxic epidermal nec-
rolysis with thalidomide therapy for myeloma. N Engl J Med 2000;343:972-3.
412. Nucera E, Schiavino D, Hohaus S, Leone G, Buonomo A, Lombardo C, et al.
Desensitization to thalidomide in a patient with multiple myeloma. Clin
Lymphoma Myeloma 2008;8:176-8.
413. Marks JG Jr, Belsito DV, DeLeo VA, Fowler JF Jr, Fransway AF, Maibach HI,
et al. North American Contact Dermatitis Group patch-test results, 1996-1998.
Arch Dermatol 2000;136:272-3.
414. Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al.
Kinase-targeted cancer therapies: progress, challenges and future directions.
Mol Cancer 2018;17:48.
415. Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Leveque D, et al. Review
of therapeutic drug monitoring of anticancer drugs, part two–targeted thera-
pies. Eur J Cancer 2014;50:2020-36.
416. Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor
receptor inhibitors. Target Oncol 2009;4:107-9.
417. Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between
skin rash and outcome in non-small-cell lung cancer patients treated with anti-
EGFR tyrosine kinase inhibitors: a literature-b ased meta-analysis of 24 trials.
Lung Cancer 2012;78:8-15.
418. Elting LS, Chang YC, Parelkar P, Boers-Doets CB, Michelet M, Hita G, et al. Risk
of oral and gastrointestinal mucosal injury among patients receiving selected tar-
geted agents: a meta-analysis. Support Care Cancer 2013;21:3243-54.
419. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications
for anti-tumor activity and management. Semin Oncol 2015;42:863-75.
420. Sugiyama
E, Umemura S, Nomura S, Kirita K, Matsumoto S, Yoh K, et al.
Impact of single nucleotide polymorphisms on severe hepatotoxicity induced
by EGFR tyrosine kinase inhibitors in patients with non-small cell lung cancer
harboring EGFR mutations. Lung Cancer 2015;90:307-13.
421. Nelson RP Jr, Cornetta K, Ward KE, Ramanuja S, Fausel C, Cripe LD.
Desensitization to imatinib in patients with leukemia. Ann Allergy Asthma
Immunol 2006;97:216-22.
422. Paolo CD. Cutaneous adverse reactions to imatinib: a case report of a suc-
cessful slow protocol for induction of drug tolerance. J Allergy Ther 2015;6:
1000203.
423. Sanchez-Lopez J, Vinolas N, Munoz-Cano R, Pascal M, Reguart N, Bartra J,
et al. Successfu l oral desensitization in a patient with hypersensitivity reaction
to crizotinib. J Investig Allergol Clin Immunol 2015;25:307-8.
424. Awad MM, Lax TP, Slawski BR, Shaw AT. Successful desensitization of two
patients with ALK-positive lung cancer and hypersensitivity to crizotinib.
J Thorac Oncol 2014;9:1726-8.
425. Bauer C, Przybilla B, Rueff F. Severe cutaneous reaction to sorafenib: in-
duction of tolerance. Acta Derm Venereol 2008;88:627-8.
426. Linauskiene K, Malinauskiene L, Vitkauskaite E, Chomiciene A, Blaziene A.
Severe adverse skin reaction and desensitization to sorafenib. Ann Allergy
Asthma Immunol 2016;117:209-10.
427. Bar-Sela G, Kedem E, Hadad S, Pollack S, Haim N, Atrash F, et al. Successful
desensitization protocol for hypersensitivity reaction caused by sunitinib in a
patient with a gastrointestinal stromal tumor. Jpn J Clin Oncol 2010;40:163-5.
428. Shirasawa M, Kubotaa M, Harada S, Niwa H, Kusuhara S, Kasajima M, et al.
Successful oral desensitization against skin rash induced by alectinib in a
patient with anaplastic lymphoma kinase-positive lung adenocarcinoma: a case
report. Lung Cancer 2016;99:66-8.
429. Brown BA, Torabi M. Incidence of infusion-associated reactions with ritux-
imab for treating multiple sclerosis: a retrospective analysis of patients treated
at a US centre. Drug Saf 2011;34:117-23.
430. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell
depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J
Med 2008;358:676-88.
431. Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al.
Rituximab in patients with primary progressive multiple sclerosis: results of a
randomized double-blind placebo-controlled multicenter trial. Ann Neurol
2009;66:460-71.
432. McLaughlin P, Hagemeister FB, Grillo-Lopez AJ. Rituximab in indolent
lymphoma: the single-agent pivotal trial. Semin Oncol 1999;26:79-87.
433. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC,
et al. Efficacy and safety of rituximab in moderately-to-severely active sys-
temic lupus erythematosus: the randomized, double-blind, phase II/III systemic
lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:
222-33.
434. RituxanÒ. In: Schwab M, editor. Encyclopedi a of Cancer. Heidelberg, Ger-
many: Springer-Verlag; 2017.
435. Brennan PJ, Rodriguez Bouza T, Hsu FI, Sloane DE, Castells MC. Hyper-
sensitivity reactions to mAbs: 105 desensitizations in 23 patients, from eval-
uation to treatment. J Allergy Clin Immunol 2009;124:1259-66.
436. Vultaggio A, Matucci A, Nencini F, Pratesi S, Petroni G, Cammelli D, et al.
Drug-specific Th2 cells and IgE antibodies in a patient with anaphylaxis to
rituximab. Int Arch Allergy Immunol 2012;159:321-6.
437. Piva E, Chieco-Bianchi F, Krajcar V, Aversa S, Plebani M. Adverse reactions
in patients with B-cell lymphomas during combined treatment with rituximab:
in vitro evaluation of rituximab hypersensitivity by basophil activation test.
Am J Hematol 2012;87:E130-1.
438. Levin AS, Otani IM, Lax T, Hochberg E, Banerji A. Reactions to rituximab in
an outpatient infusion center: a 5-year review. J Allergy Clin Immunol Pract
2017;5:107-113.e1.
439. Hong DI, Bankova L, Cahill KN, Kyin T, Castells MC. Allergy to monoclonal
antibodies: cutting-edge desensitization methods for cutting-edge therapies.
Expert Rev Clin Immunol 2012;8:43-52.
440. Hong DI, Dioun AF. Indications, protocols, and outcomes of drug de-
sensitizations for chemotherapy and monoclonal antibodies in adults and
children. J Allergy Clin Immunol Pract 2014;2:13-9.
441. Lebel E, Ben-Yehuda D, Bohbot E, Dranitzki Z, Shalit M, Tal Y. Hypersen-
sitivity reactions to rituximab: 53 successful desensitizations in 7 patients with
severe, near-fatal reactions. J Allergy Clin Immunol Pract 2016;4:1000-2.
442. Wong JT, Long A. Rituximab hypersensitivi ty: evaluation, desensitization, and
potential mechanisms. J Allergy Clin Immunol Pract 2017;5:1564-71.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S106 BROYLES ET AL
443. Caimmi SM, Caimmi D, Riscassi S, Marseglia GL. A new pediatric protocol
for rapid desensitization to monoclonal antibodies. Int Arch Allergy Immunol
2014;165:214-8.
444. Dilley MA, Lee JP, Platt CD, Broyles AD. Rituximab desensitization in pe-
diatric patients: results of a case series. Pediatr Allergy Immunol Pulmonol
2016;29:91-4.
445. Matucci A, Pratesi S, Petroni G, Nencini F, Virgili G, Milla M, et al. Aller-
gological in vitro and in vivo evaluation of patients with hypersensitivity re-
actions to in fl iximab. Clin Exp Allergy 2013;43:659-64.
446. O’Neil BH, Allen R, S pigel DR, Stinchcombe TE, Moore DT, Berlin JD,
et al. High incidence of ce tuximab-relate d infusion reactions in Tennes see
and North Carolina and the association with atopic history. J Clin Oncol
2007;25:3644-8.
447. Arnold DF, Misbah SA. Cetuximab-induced anaphylaxis and IgE specific for
galactose-alpha-1,3-galactose. N Engl J Med 2008;358:2735, author reply
2735-6.
448. Slavin R. Faculty of 1000 Evaluation for the Relevance of Tick Bites to the
Production of IgE Antibodies to the Mammalian Oligosaccharide Galactose-
alpha-1,3-galactose. London, UK: Faculty of 1000, Ltd; 2011.
449. Sicherer S, Wang J. Faculty of 1000 evaluation for delayed anaphylaxis,
angioedema, or articaria after c onsumption o f red meat in patien ts with
IgE antibodies specific for galactose-alpha-1,3-galactose. London, UK:
Faculty of 1000, Ltd; 2009.
450. Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meat-induced
anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE
confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol 2009;
124:603-5.
451. Jerath MR, Kwan M, Kannarkat M, Mirakhur B, Carey L, Valgus J, et al.
A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol
2009;123:260-2.
452. Gernez Y, Freeman AF, Ho lland SM, Garabedian E, Patel NC, Puck JM, et al.
Autosomal dominant hyper-IgE syndrome in the USIDNET Registry. J Allergy
Clin Immunol Pract 2018;6:996-1001.
453. Modena BD, White AA. Can diet modification be an effective treatment in
aspirin-exacerbated respiratory disease? J Allergy Clin Immunol Pract 2018;6:
832-3.
454. Gergen PJ. Rethinking access to care. J Allergy Clin Immunol Pract 2018;6:
853-4.
455. Rejnö G, Lundholm C, Larsson K, Larsson H, Lichtenstein P,
D’Onofrio BM, e t al. Adverse pregnancy outcomes in asthmatic women: a
population-based family design study. J Allergy Clin Immunol Pract 2018;6:
916-922.e6.
456. Zeiger RS, Tran TN, Butler RK, Schatz M, Li Q, Khatry DB, et al. Rela-
tionship of blood eosinophil count to exacerbations in chronic obstructive
pulmonary disease. J Allergy Clin Immunol Pract 2018;6:944-954.e5.
457. Choquette D, Faraawi R, Chow A, Rodrigues J, Bensen WJ, Nantel F. Inci-
dence and management of infusion reactions to infliximab in a prospective
real-world community registry. J Rheumatol 2015;42:1105-11.
458. Ducharme J, Pelletier C, Zacharias R. The safety of infliximab infusions in the
community setting. Can J Gastroenterol 2010;24:307-11.
459. Mourad AA, Boktor MN, Yilmaz-Demirdag Y, Bahna SL. Adverse reactions
to infliximab and the outcome of desensitization. Ann Allergy Asthma
Immunol 2015;115:143-6.
460. Feuerstein JD, Cheifetz AS. Miscellaneous adverse events with biologic agents
(excludes infection and malignancy). Gastroenterol Clin North Am 2014;43:
543-63.
461. Zeltser R, Valle L, Tanck C, Holyst MM, Ritchlin C, Gaspari AA. Clinical,
histological, and immunophenotypic characteristics of injection site reactions
associated with etanercept: a recombinant tumor necrosis factor alpha receptor:
Fc fusion protein. Arch Dermatol 2001;137:893-9.
462. Bavbek S, Ataman S, Akinci A, Castells M. Rapid subcutaneous desensiti-
zation for the management of local and systemic hypersensitivity reactions to
etanercept and adalimumab in 12 patients. J Allergy Clin Immunol Pract 2015;
3:629-32.
463. Gamarra RM, McGraw SD, Drelichman VS, Maas LC. Serum sickness-like
reactions in patients receiving intravenous infliximab. J Emerg Med 2006;30:
41-4.
464. Cleynen I, Van Moerkercke W, Billiet T, Vandecandelaere P, Vande
Casteele N, Breynaert C, et al. Characteristics of skin lesions associated with
anti-tumor necrosis factor therapy in patients with inflammatory
bowel disease:
a cohort study. Ann Intern Med 2016;164:10-22.
465. Bulur I, Keseroglu HO, Saracoglu ZN, Gonul M. Symmetrical drug-related
intertriginous and flexural exanthema (Baboon syndrome) associated with
infliximab. J Dermatol Case Rep 2015;9:12-4.
466. Mounach A, Rezqi A, Nouijai A, Ghozlani I, Achemlal L, Maghraoui AE,
et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheu-
matoid arthritis disease. Rheumatol Int 2013;33:1351-3.
467. Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L,
et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233
cases. Medicine (Baltimore) 2007;86:242-51.
468. Vergara G, Silvestre JF, Betlloch I, Vela P, Albares MP, Pascual JC. Cuta-
neous drug eruption to infliximab: report of 4 cases with an interface dermatitis
pattern. Arch Dermatol 2002;138:1258-9.
469. Freling E, Peyrin-Biroulet L, Poreaux C, Morali A, Waton J, Schmutz JL, et al.
IgE antibodies and skin tests in immediate hypersensitivity reactions to
infliximab in inflammatory bowel disease: impact on infliximab retreatment.
Eur J Gastroenterol Hepatol 2015;27:1200-8.
470. Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D,
et al. Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy
and monoclonal antibodies. J Allergy Clin Immunol Pract 2016;4:497-504.
471. Vultaggio A, Matucci A, Nencini F, Pratesi S, Parronchi P, Rossi O, et al.
Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related
severe anaphylactic reactions. Allergy 2010;65:657-61.
472. Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal
antibodies again st tumor necrosis factor used in chronic immune-mediated
Inflammatory conditions: systematic review and meta-analysis. JAMA Intern
Med 2013;173:1416-28.
473. O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion
reactions in patients with inflammatory bowel disease: a systematic review and
meta-analysis. Inflamm Bowel Dis 2014;20:1-6.
474. Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, Lynch DM,
Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal
antibodies: phenotypes and endotypes. J Allergy Clin Immuno l 2018;142:
159-170.e2.
475. Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, et al. The
incidence and management of infusion reactions to infliximab: a large center
experience. Am J Gastroenterol 2003;98:1315-24.
476. Picard M, Galvao VR. Current knowledge and management of hypersensitivity
reactions to monoclonal antibodies. J Allergy Clin Immunol Pract 2017;5:
600-9.
477. Ben-Horin S, Yavzori M, Katz L, Kopylov U, Picard O, Fudim E, et al. The
immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the
intact infliximab molecule is more clinically useful. Gut 2011;60:41-8.
478. Steenholdt C, Svenson M, Bendtzen K, Thomsen OO, Brynskov J,
Ainsworth MA. Acute and delayed hypersensitivity reactions to infl iximab and
adalimumab in a patient with Crohn’s disease. J Crohns Colitis 2012;6:108-11.
479. Miheller P, Muzes G, Lakatos G, Mihaly E, Tulassay Z. Repeated in fl iximab
therapy after serum sickness-like reaction in Crohn’s
disease. J Emerg Med
2007;32:209-10, author reply 210.
480. Kattan M, Bacharier LB, O’Connor GT, Cohen R, Sorkness RL, Morgan W,
et al. Spirometry and impulse oscillometry in preschool children: acceptability
and relationship to maternal smoking in pregnancy. J Allergy Clin Immunol
Pract 2018;6:1596-1603.e6.
481. Korycka-Wolowiec A, Wolowiec D, Robak T. Ofatumumab for treating
chronic lymphocytic leukemia: a safety profile. Expert Opin Drug Saf 2015;14:
1945-59.
482. Skirvin JA, Kowalczyk R. Obinutuzumab (GazyvaÒ). Oncology Times 2017;
39:21.
483. Dunlop JH, Keet CA, Mudd K, Wood RA. Long-term follow-up after baked
milk introduction. J Allergy Clin Immunol Pract 2018;6:1699-704.
484. Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Pete rsen J, Chang DJ.
Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-
naive, rheumatoid arthritis patients with an inadequate response to metho-
trexate: a randomised, double-blind, placebo-controlled clinical trial. Ann
Rheum Dis 2011;70:2119-25.
485. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al.
Obinutuzumab plus chlorambucil in patients with CLL and coexisting condi-
tions. N Engl J Med 2014;370:1101-10.
486. Morgan BW, Siddharthan T, Grigsby MR, Pollard SL, Kalyesubula R,
Wise RA, et al. Asthma and allergic disorders in Uganda: a population-based
study across urban and rural settings. J Allergy Clin Immunol Pract 2018;6:
1580-7.e2.
487. Arora A, Bhatt VR, Liewer S, Armitage JO, Bociek RG. Brentuximab vedotin
desensitization in a patient with refractory Hodgkin’s lymphoma. Eur J Hae-
matol 2015;95:361-4.
488. DeVita MD, Evens AM, Rosen ST, Greenberger PA, Petrich AM. Multiple
successful desensitizations to brentuximab vedotin: a case report and literature
review. J Natl Compr Canc Netw 2014;12:465-71.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S107
489. O’Connell AE, Lee JP, Yee C, Kesselheim J, Dioun A. Successful desensiti-
zation to brentuximab vedotin after anaphylaxis. Clin Lymphoma Myeloma
Leuk 2014;14:e73-5.
490. NICE rules that PCTs must make Herceptin available. Nurse Prescribing 2006;
4:310-1.
491. Cook-Bruns N. Retrospective analysis of the safety of Herceptin immuno-
therapy in metastatic breast cancer. Oncology 2001;61:58-66.
492. Thompson LM, Eckmann K, Boster BL, Hess KR, Michaud LB, Esteva FJ,
et al. Incidence, risk factors, and management of infusion-related reactions in
breast cancer patients receiving trastuzumab. Oncologist 2014;19:228-34.
493. Sheu J, Hawryluk EB, Litsas G, Thakuria M, LeBoeuf NR. Papulopustular
acneiform eruptions resulting from trastuzumab, a HER2 inhibitor. Clin Breast
Cancer 2015;15:e77-e81.
494. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus
trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;
366:109-19.
495. Product update: Perjeta extended indication in breast cancer. The Pharma-
ceutical Journal. August 21, 2015. Available from: https://www.
pharmaceutical-journal.com/news-and-analysis/notice-board/perjeta-extended-
indication-in-breast-cancer/20069160 .article. Accessed July 30, 2020.
496. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al.
Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast
cancer. N Engl J Med 2015;372:724-34.
497. Gonzalez-de-Olano D, Morgado JM, Juarez-Guerrero R, Sanchez-Munoz L,
Letellez-Fernandez J, Malon-Gimenez D, et al. Positive basophil activation test
following anaphylaxis to pertuzumab and successful treatment with rapid
desensitization. J Allergy Clin Immunol Pract 2016;4:338-40.
498. Wang J-Y, Yao T-C, Tsai Y-T, Wu AC, Tsai H-J. Increased dose and duration
of statin use is associated with decreased asthma-related emergency department
visits and hospitalizations. J Allergy Clin Immunol Pract 2018;6:1588-15895.
e1.
499. Song X, Long SR, Barber B, Kassed CA, Healey M, Jones C, et al. Systematic
review on infusion reactions associated with chemotherapies and
monoclonal antibodies for metastatic colorectal cancer. Curr Clin Pharmacol
2012;7:56-65.
500. Reidy DL, Chung KY, Timoney JP, Park VJ, Hollywood E, Sklarin NT, et al.
Bevacizumab 5 mg/kg can be infused safely over 10 minutes. J Clin Oncol
2007;25:2691-5.
501. Sun A, Alshuaibi W, Petroni D, Skoda-Smith S, Goldberg MJ, Hale S. Im-
mune modulation in a patient with Morquio syndrome treated with enzyme
replacement therapy. J Allergy Clin Immunol Pract 2018;6:1749-51.
502. Justet A, Neukirch C, Poubeau P, Arrault X, Borie R, Dombret MC, et al.
Successful rapid tocilizumab desensitization in a patient with Still disease.
J Allergy Clin Immunol Pract 2014;2:631-2.
503. Rocchi V, Puxeddu I, Cataldo G, Del Corso I, Tavoni A, Bazzichi L, et al.
Hypersensitivity reactions to tocilizumab: role of skin tests in diagnosis.
Rheumatology (Oxford) 2014;53:1527-9.
504. Ben-Yakov G, Kapuria D, Marko J, Cho MH, Pittaluga S, Kleiner DE, et al.
Liver disturbances in activated phosphoinositide 3-kinase
d
syndrome. J Al-
lergy Clin Immunol Pract 2018;6:1763-5.
505. Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV.
American Academy of Allergy, Asthma & Immunology/American College of
Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-
associated anaphylaxis. J Allergy Clin Immunol 2007;120:1373-7.
506. Lieberman PL, Umetsu DT, Ca rrigan GJ, Rahmaoui A. Anaphylactic reactions
associated with omalizumab administration: analysis of a case-control study.
J Allergy Clin Immunol 2016;138:913-915.e2.
507. Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted
progression of anaphylaxis after omalizumab administration in patients with
asthma. J Allergy Clin Immunol 2007;120:1378-81.
508. Lieberman P, Rahmaoui A, Wong DA. The safety and interpretability of skin
tests with omalizumab. Ann Allergy Asthma Immunol 2010;105:493-5.
509. Cox L, Lieberman P, Wallace D, Simons FE, Finegold I, Platts-Mills T, et al.
American Academy of Allergy, Asthma & Immunology/American College of
Allergy, Asthma & Immunology Omalizumab-Associated Anaphylaxis Joint
Task Force follow-up report. J Allergy Clin Immunol 2011;128:210-2.
510. Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omali-
zumab administration after successful long-term therapy. Allergy Asthma Proc
2007;28:313-9.
511. Owens G, Petrov A. Successful desensitization of three patients with hyper-
sensitivity reactions to omalizumab. Curr Drug Saf 2011;6:339-42.
512. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH Jr. Effect of long-
term exposure to insulin lispro on the induction of antibody response in pa-
tients with type 1 or type 2 diabetes. Diabetes Care 2003;26:89-96.
513. Blanco C, Castillo R, Quiralte J, Delgado J, Garcia I, de Pablos P, et al.
Anaphylaxis to subcutaneous neutral protamine Hagedorn insulin with
simultaneous sensitization to protamine and insulin. Allergy 1996;51:421-4.
514. Fernandez L, Duque S, Montalban C, Bartolome B. Allergy to human insulin.
Allergy 2003;58:1317.
515. Perez E, Gonzalez R, Martinez J, Iglesias J, Matheu V. Detemir insulin-
induced anaphylaxis. Ann Allergy Asthma Immunol 2009;102:174-5.
516. Silva ME, Mendes MJ, Ursich MJ, Rocha DM, Brito AH, Fukui RT, et al.
Human insulin allergy—immediate and late type III reactions in a long-
standing IDDM patient. Diabetes Res Clin Pract 1997;36:67-70.
517. Blumer IR. Severe injection site reaction to insulin detemir. Diabetes Care
2006;29:946.
518. Mandrup-Poulsen T, Molvig J, Pildal J, Rasmussen AA, Andersen L,
Skov BG, et al. Leukocytoclastic vasculi tis induced by subcutaneous injection
of human insulin in a patient with type 1 diabetes and essential thrombocy-
temia. Diabetes Care 2002;25:242-3.
519. Kim D, Baraniuk J. Delayed-type hypersensitivity reaction to the meta-cresol
component of insulin. Ann Allergy Asthma Immunol 2007;99:194-5.
520. Kollner A, Senff H, Engelmann L, Kalveram KJ, Velcovsky HG, Haneke E.
Delayed hypersensitivity to protamine and immediate hypersensitivity to in-
sulin [in German]. Deutsch Med Wochenschr 1991;116:1234-8.
521. Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131
metabolism in hum an subjects: demonstration of insulin binding globulin in
the circulation of insulin treated subjects. J Clin Invest 1956;35:170-90.
522. Bodtger U, Wittrup M. A rational clinical approach to suspected insulin al-
lergy: status after five years and 22 cases. Diabetic Med 2005;22:102-6.
523. Clerx V, Van Den Keybus C, Kochuyt A, Goossens A. Drug intolerance re-
action to insulin therapy caused by metacresol. Contact Dermatitis 2003;48:
162-3.
524. Dykewicz MS, Kim HW, Orfan N, Yoo TJ, Lieberman P. Immunologic
analysis of anaphylaxis to protamine component in neutral protamine Hage-
dorn human insulin. J Allergy Clin Immunol 1994;93:117-25.
525. Feinglos MN, Jegasothy BV. “Insulin” allergy due to zinc. Lancet 1979;1:
122-4.
526. Stewart WJ, McSweeney SM, Kellett MA, Faxon DP, Ryan TJ. Increased risk
of severe protamine reactions in NPH insulin-dependent diabetics undergoing
cardiac catheterization. Circulation 1984;70:788-92.
527. Chen YM, Huang H. Allergy to soft cannula of insulin pump in diabetic pa-
tient. Pakistan J Med Sci 2017;33:245-7.
528. Roest MA, Shaw S, Orton DI. Insulin-injection-site reactions associated with
type I latex allergy. N Engl J Med 2003;348:265-6.
529. Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL,
Krasner AS. Immunological responses to exogenous insulin. Endocrine Rev
2007;28:625-52.
530. Horrow JC, Pharo GH, Levit LS, Freeland C. Neither skin tests nor serum
enzyme-linked immunosorbent assay tests provide specificity for protamine
allergy. Anesth Analg 1996;82:386-9.
531. Weiss ME, Nyhan D, Peng ZK, Horrow JC, Lowenstein E, Hirshman C, et al.
Associatio
n of protamine IgE and IgG antibodies with life-threatening re-
actions to intravenous protamine. N Engl J Med 1989;320:886-92.
532. Lee AY, Chey WY, Choi J, Jeon JS. Insulin-induced drug eruptions and
reliability of skin tests. Acta Derm Venereol 2002;82:114-7.
533. Kawanami D, Ito T, Watanabe Y, Kinoshita J, Sakamoto M, Isaka T, et al.
Successful control of a case of severe insulin allergy with liraglutide.
J Diabetes Investig 2013;4:94-6.
534. Castera V, Dutour-Meyer A, Koeppel M, Petitjean C, Darmon P. Systemic
allergy to human insulin and its rapid and long acting analogs: successful
treatment by continuous subcutaneous insulin lispro infusion. Diabetes Metab
2005;31:391-400.
535. Eapen SS, Connor EL, Gern JE. Insulin desensitization with insulin lispro and
an insulin pump in a 5-year-old child. Ann Allergy Asthma Immunol 2000;85:
395-7.
536. Matheu V, Perez E, Hernandez M, Diaz E, Darias R, Gonzalez A, et al. Insulin
allergy and resistance successfully treated by desensitisation with Aspart in-
sulin. Clin Mol Allergy CMA 2005;3:16.
537. Wheeler BJ, Taylor BJ. Successful management of allergy to the insulin
excipient metacresol in a child with type 1 diabetes: a case report. J Med Case
Rep 2012;6:263.
538. Yong PF, Malik R, Arif S, Peakman M, Amiel S, Ibrahim MA, et al. Ritux-
imab and omalizumab in severe, refractory insulin allergy. N Engl J Med 2009;
360:1045-7.
539. Murray BR, Jewell JR, Jackson KJ, Agboola O, Alexander BR, Sharma P.
Type III hypersensitivity reaction to subcutaneous insulin preparations in a
type 1 diabetic. J Gen Intern Med 2017;32:841-5.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S108 BROYLES ET AL
540. Bayraktar F, Akinci B, Demirkan F, Yener S, Yesil S, Kirmaz C, et al. Serum
sickness-like reactions associated with type III insulin allergy responding to
plasmapheresis. Diabetic Med 2009;26:659-60.
541. Buchheit KM, Bernstein JA. Progestogen hypersensitivity: heterogeneous
manifestations with a common trigger. J Allergy Clin Immunol Pract 2017;5:
566-74.
542. Foer D, Buchheit KM, Gargiulo AR, Lynch DM, Castells M, Wickner PG.
Progestogen hypersensitivity in 24 cases: diagnosis, management, and pro-
posed renaming and classification. J Allergy Clin Immunol Pract 2016;4:
723-9.
543. Prieto-Garcia A, Sloane DE, Gargiulo AR, Feldweg AM, Castells M. Auto-
immune progesterone dermatitis: clinical presentation and management with
progesterone desensitization for successful in vitro fertilization. Fertil Steril
2011;95:1121.e9-e13.
544. Cocuroccia B, Gisondi P, Gubinelli E, Girolomoni G. Autoimmune proges-
terone dermatitis. Gynecol Endocrinol 2006;22:54-6.
545. Asai J, Katoh N, Nakano M, Wada M, Kishimoto S. Case of autoimmune
progesterone dermatitis presenting as fixed drug eruption. J Dermatol 2009;36:
643-5.
546. Bernstein IL, Bernstein DI, Lummus ZL, Bernstein JA. A case of progester-
one-induced anaphylaxis, cyclic urticaria/angioedema, and autoimmune
dermatitis. J Womens Health (Larchmt) 2011;20:643-8.
547. Wintzen M, Goor-van Egmond MB, Noz KC. Autoimmune progesterone
dermatitis presenting with purpura and petechiae. Clin Exp Dermatol 2004;29:
316.
548. Snyder JL, Krishnaswamy G. Autoimmune progesterone dermatitis and its
manifestation as anaphylaxis: a case report and literature review. Ann Allergy
Asthma Immunol 2003;90:469-77.
549. Li RC, Buchheit KM, Bernstein JA. Progestogen hypersensitivity. Curr Al-
lergy Asthma Rep 2018;18:1.
550. Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with
endometriosis: case report and review of the literature. Clin Mol Allergy CMA
2004;2:10.
551. Heffler E, Fichera S, Nicolosi G, Crimi N. Anaphylaxis due to progesterone
hypersensitivity successfully treated with omalizumab. J Allergy Clin Immunol
Pract 2017;5:852-4.
552. Shahar E, Bergman R, Pollack S. Autoimmune progesterone dermatitis:
effective prophylactic treatment with danazol. Int J Dermatol 1997;36:708-11.
553. Wojnarowska F, Greaves MW, Peachey RD, Drury PL, Besser GM. Proges-
terone-induced erythema multiforme. J R Soc Med 1985;78:407-8.
554. Medeiros S, Rodrigues-Alves R, Costa M, Afonso A, Rodrigues A, Cardoso J.
Autoimmune progesterone dermatitis: treatment with oophorectomy. Clin Exp
Dermatol 2010;35:e12-e13.
555. Johnson KP. Glat iramer acetate for treatment of relapsing-remitting multiple
sclerosis. Expert Rev Neurother 2012;12:371-84.
556. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer
acetate in multiple sclerosis. Drug Saf 2001;24:979-90.
557. Bosca I, Bosca M, Belenguer A, Evole M, Hernandez M, Casanova B, et al.
Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol
2006;253:1370-1.
558. Cicek D, Kandi B, Oguz S, Cobanoglu B, Bulut S, Saral Y. An urticarial
vasculitis case induced by glatiramer acetate. J Dermatolog Treat 2008;19:
305-7.
559. Feldmann R, Schierl M, Rauschka H, Sator PG, Breier F, Steiner A. Necro-
tizing skin lesions with involvement of muscle tissue after subcutaneous in-
jection of glatiramer acetate. Eur J Dermatol 2009;19:385.
560. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous
injection of glatiramer acetate. J Dtsch Dermatol Ges 2007;5:1122-3.
561. Kluger N, Thouvenot E, Camu W, Guillot B. Cutaneous adverse events related
to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol
Venereol 2009;23:1332-3.
562. Koller S, Kranke B. Nicolau syndrome following subcutaneous glatiramer-
acetate injection. J Am Acad Dermatol 2011;64:e16-7.
563. Thouvenot E, Hillaire-Buys D, Bos-Thompson MA, Rigau V, Durand L,
Guillot B, et al. Erythema nodosum and glatiramer acetate treatment in re-
lapsing-remitting multiple sclerosis. Mult Scler 2007;13:941-4.
564. Baumgartner A, Stich O, Rauer S. Anaphylactic reaction after injection of
glatiramer acetate (Copaxone(R)) in patients with relapsing-remitting multiple
sclerosis. Eur Neurol 2011;66:368-70.
565. Mayorga C, Blazquez AB, Dona I, Gomez F, Chaves P, Sanchez-
Quintero MJ, et al. Immunological mechanisms underlying delayed-type
hypersensitivity reactions to glatiramer acetate. Ann Allergy Asthma
Immunol 2012;109:47-51.
566. Rauschka H, Farina C, Sator P, Gudek S, Breier F, Schmidbauer M. Severe
anaphylactic reaction to glatiramer acetate with specific IgE. Neurology 2005;
64:1481-2.
567. Soriano Gomis V, Perez Sempere A, Gonzalez Delgado P, Sempere JM,
Niveiro
Hernandez E, Marco FM. Glatiramer acetate anaphylaxis: detection of
antibodies and basophil activation test. J Investig Allergol Clin Immunol 2012;
22:65-6.
568. Crestani E, Lee J, Gorman M, Castells M, Dioun Broyles AF. IgE-mediated
hypersensitivity reaction and desensitization to glatiramer acetate in a pediatric
patient. Pediatr Allergy Immunol 2014;25:821-3.
569. Sanchez-Lopez J. Allergy workup in immediate-type local reactions to gla-
tiramer acetate. J Investig Allergol Clin Immunol 2010;20:521-3.
570. Syrigou E, Psarros P, Grapsa D, Syrigos K. Successful rapid desensitization to
glatiramer acetate in a patient with multiple sclerosis. J Investig Allergol Clin
Immunol 2015;25:214-5.
571. Bains SN, Hsieh FH, Rensel MR, Radojicic C, Katz HT, Inamdar SR, et al.
Glatiramer acetate: successful desensitization for treatment of multiple scle-
rosis. Ann Allergy Asthma Immunol 2010;104:321-5.
572. Sheth SS, Posner MA. Modifi ed protocol for desensitization to glatiramer
acetate. Ann Allergy Asthma Immunol 2010;105:190.
573. Genezyme Corporation. Guideline to re-challenge administration of Cerezyme
(imiglucerase for injection). Cerezyme Monogr 2010:1-10.
574. Peroni DG, Pescollderungg L, Piacentini GL, Cassar W, Boner AL. Effective
desensitization to imiglucerase in a patient with type I Gaucher disease.
J Pediatr 2009;155:940-1.
575. Zhao H, Bailey LA, Grabowski GA. Enzyme therapy of gaucher disease:
clinical and biochemical changes during production of and tolerization for
neutralizing antibodies. Blood Cells Mol Dis 2003;30:90-6.
576. Brooks DA, Kakavanos R, Hopwood JJ. Significance of immune response to
enzyme-replacement therapy for patients with a lysosomal storage disorder.
Trends Mol Med 2003;9:450-3.
577. Starzyk K, Richards S, Yee J, Smith SE, Kingma W. The long-term interna-
tional safety experience of imiglucerase therapy for Gaucher disease. Mol
Genet Metab 2007;90:157-63.
578. Erdogdu D, Gelincik A, Canbaz B, Colakoglu B, Buyukozturk S, Tanakol R.
Successful desensitization to imiglucerase of an adult patient diagnosed with
type I Gaucher disease. Int Arch Allergy Immunol 2013;160:215-7.
579. Tsilochristou O, Gkavogiannakis NA, Ioannidou EN, Makris M. Successful
rapid desensitization to imiglucerase in an adult patient with Gaucher disease
and documented IgE-mediated hypersensitivity. J Allergy Clin Immunol Pract
2015;3:624-6.
580. Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al.
Surveillance for safety after immunization: Vaccine Adverse Event Reporting
System (VAERS)–United States, 1991-2001. MMWR Surveill Summ 2003;
52:1-24.
581. McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP,
et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy
Clin Immunol 2016;137:868-78.
582. Zent O, Arras-Reiter C, Broeker M, Hennig R. Immediate allergic reactions after
vaccinations–a post-marketing surveillance review. Eur J Pediatr 2002;161:21-5.
583. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA,
Branum A, et al. Second symposium on the definition and management of
anaphylaxis: summary report–Second National Institute of Allergy and In-
fectious Disease/Food Allergy and Anaphylaxis Network symposium.
J Allergy Clin Immunol 2006;117:391-7.
584. Wood RA, Berger M, Dreskin SC, Setse R, Engler RJ, Dekker CL, et al. An
algorithm for treatment of patients with hypersensitivity reactions after vac-
cines. Pediatrics 2008;122:e771-e777.
585. General recommendations on immunization—recommendations of the Advi-
sory Committee on Immunization Practices (ACIP). MMWR Recomm Rep
2011;60:1-64.
586. Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J,
et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy
Clin Immunol 2012;130:25-43.
587. Blanco C, Carrillo T, Ortega N, Alvarez M, Dominguez C, Castillo R. Com-
parison of skin-prick test and specific serum IgE determination for the diag-
nosis of latex allergy. Clin Exp Allergy 1998;28:971-6.
588. Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with
systemic immediate-type reactions, including anaphylaxis, to vaccines.
J Allergy Clin Immunol 1996;98:1058-61.
589. Greenhawt MJ, Spergel JM, Rank MA, Green TD, Mansoor D, Sharma H,
et al. Safe administration of the seasonal trivalent influenza vaccine to children
with severe egg allergy. Ann Allergy Asthma Immunol 2012;109:426-30.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S109
590. Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M, SNIF-
FLE Study Investigators. Safety of live attenuated influenza vaccine in atopic
children with egg aller gy. J Alle rgy Clin Immunol 2015;136:376-81.
591. Fisher MM, More DG. The epidemiology and clinical features of anaphylactic
reactions in anaesthesia. Anaesth Intensive Care 1981;9:226-34.
592. Galletly DC, Treuren BC. Anaphylactoid reactions during anaesthesia: seven
years’ experience of intradermal testing. Anaesthesia 1985;40:329-33.
593. Hatton F, Tiret L, Maujol L, N’Doye P, Vourc’h G, Desmonts JM, et al.
INSERM. Epidemiological survey of anesthesia. Initial results [in French].
Ann Fr Anesth Reanim 1983;2:331-86.
594. Watkins J. Adverse anaesthetic reactions: an update from a proposed national
reporting and advisory service. Anaesthesia 1985;40:797-800.
595. Laxenaire MC. Epidemiology of anesthetic anaphylactoid reactions: fourth
multicenter survey (July 1994-December 1996) [in French]. Ann Fr Anesth
Reanim 1999;18:796-809.
596. Whittington T, Fisher MM. Anaphylactic and anaphylactoid reactions. Bail-
liere’s Clin Anaesthesiol 1998;12:301-23.
597. Gibbs NM, Sadleir PH, Clarke RC, Platt PR. Survival from perioperative
anaphylaxis in Western Australia 2000-2009. Br J Anaesth 2013;111:589-93.
598. Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, Marinho S, et al.
Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and
clinical features of perioperative anaphylaxis in the 6th National Audit Project
(NAP6). Br J Anaesth 2018;121:159-71.
599. Dong SW, Mertes PM, Petitpain N, Hasdenteufel F, Malinovsky JM. GERAP.
Hypersensitivity reactions during anesthesia: results from the ninth French
survey (2005-2007). Minerva Anestesiol 2012;78:868-78.
600. Gurrieri C, Weingarten TN, Martin DP, Babovic N, Narr BJ, Sprung J, et al.
Allergic reactions during anesthesia at a large United States referral center.
Anesth Analg 2011;113:1202-12.
601. Harboe T, Guttormsen AB, Irgens A, Dybendal T, Florvaag E. Anaphylaxis
during anesthesia in Norway: a 6-year single-center follow-up study. Anes-
thesiology 2005;102:897-903.
602. Lobera T, Audicana MT, Pozo MD, Blasco A, Fernandez E, Canada P, et al.
Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain.
J Investig Allergol Clin Immunol 2008;18:350-6.
603. Dewachter P, Mouton-Faivre C, Hepner DL. Perioperative anaphylaxis: what
should be know n? Curr Allergy Asthma Rep 2015;15:21.
604. The Association of Anaesthetists. Suspected anaphylactic reactions associated
with anaesthesia. London, UK: The Association of Anaesthetists of Great Britain
and Ireland and British Society for Allergy and Clinical Immunology; 2003.
Available from: https://anaesthetists.org/H ome/Resources-publications/
Guidelines/Archived-guidelines. Accessed July 30, 2020.
605. Currie M, Webb RK, Williamson JA, Russell WJ, Mackay P. The Australian
Incident Monitoring Study. Clinical anaphylaxis: an analysis of 2000 incident
reports. Anaesth Intensive Care 1993;21:621-5.
606. Lienhart A, Auroy Y, Pequignot F, Benhamou D, Warszawski J, Bovet M,
et al. Survey of anesthesia-related mortality in France. Anesthesiology 2006;
105:1087-97.
607. Mitsuhata H, Hasegawa J, Matsumoto S, Ogawa R. The epidemiology and
clinical features of anaphylactic and anaphylactoid reactions in the perioper-
ative period in Japan: a survey with a questionnaire of 529 hospitals approved
by Japan Society of Anesthesiology [in Japanese]. Masui 1992;41:1825-31.
608. Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia:
controversies and new insights. Anesthesiology 2009;111:1141-50.
609. Harper NJ, Dixon T, Dugue P, Edgar DM, Fay A, Gooi HC, et al. Suspected
anaphylactic reactions associated with anaesthesia. Anaesthesia 2009;64:
199-211.
610. Kroigaard M, Garvey LH, Gillberg L, Johansson SG, Mosbech H, Florvaag E,
et al. Scandinavian Clinical Practice Guidelines on the diagnosis, management
and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand
2007;51:655-70.
611. Societe Francaise d’anesthesie et Reanimation, Societe Francaise d’allergolo-
gie. Reducing the risk of anaphylaxis during anaesthesia [in French]. Ann Fr
Anesth Reanim 2011;30:212-22.
612. ANZAAG anaphylaxis management guidelines. Australian & New Zealand
Anaesthetic Allergy Group. 2016. Available from: http://www.anzaag.com/
Mgmt%20Resources.aspx. Accessed July 30, 2020.
613. Kolawole H, Marshall SD, Crilly H, Kerridge R, Roessler P. Australian and
New Zealand Anaesthetic Allergy Group/Australian and New Zealand College
of Anaesthetists Perioperative Anaphylaxis Management Gu idelines. Anaesth
Intensive Care 2017;45:151-8.
614. Schumacher J. Fatal anaphylaxis to atracurium: a case report. A A Pract 2019;
12:145-6.
615. Dewachte
r P, Mouton-Faivre C, Emala CW, Beloucif S. Case scenario:
bronchospasm during anesthetic induction. Anesthesiology 2011;114:1200- 10.
616. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP,
Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to
sudden emotional stress. N Engl J Med 2005;352:539-48.
617. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. In-
ternational Expert Consensus Document on Takotsubo syndrome (Part I):
clinical charact eristics, diagnostic criteria, and pathophysiology. Eur Heart J
2018;39:2032-46.
618. Litvinov IV, Kotowycz MA, Wassmann S. Iatrogenic epinephrine-induced
reverse Takotsubo cardiomyopathy: direct evidence supporting the role of
catecholamines in the pathophysiology of the “broken heart syndrome”. Clin
Res Cardiol 2009;98:457-62.
619. Dewachter P, Tanase C, Levesque E, Nicaise-Roland P, Chollet-Martin S,
Mouton-Faivre C, et al. Apical ballooning syndrome following perioperative
anaphylaxis is likely related to high doses of epinephrine. J Anesth 2011;25:
282-5.
620. Y-Hassan S. Clinical features and outcome of epinephrine-induced takotsubo
syndrome: analysis of 33 publishedcases. Cardiovasc RevascMed2016;17:450-5.
621. Suk EH, Kim DH, Kweon TD, Na SW, Shin JA. Stress-induced cardiomy-
opathy following cephalosporin-induced anaphylactic shock during general
anesthesia. Can J Anaesth 2009;56:432-6.
622. Dewachter P, Tchotourian S, Nicaise-Roland P, Fargeot C, Escalup R. Peri-
operative anaphylaxis to ethylene oxide: twenty years after, a diagnosis
revisted. J Allergy Ther 2010;1:1000102.
623. Maciag MC, Nargozian C, Broyles AD. Intraoperative anaphylaxis secondary
to systemic cooling in a pediatric patient with cold-induced urticaria. J Allergy
Clin Immunol Pract 2018;6:1394-5.
624. Hermite L, Louvier N, Hilaire P, Orry D, Seltzer S, Collet E. Neostigmine
induced anaphylaxis in the wake of surgery. Anaesth Crit Care Pain Med 2015;
34:109-11.
625. Ue KL, Kasternow B, Wagner A, Rutkowski R, Rutkowski K. Sugammadex:
an emerging trigger of intraoperative anaphylaxis. Ann Allergy Asthma
Immunol 2016;117:714-6.
626. Dewachter P, Mouton-Faivre C, Castells MC, Hepner DL. Anesthesia in the
patient with multiple drug allergies: are all allergies the same? Curr Opin
Anaesthesiol 2011;24:320-5.
627. Baldo BA. Relevance and value of a morphine immunoassay as a diagnostic
aid for neuromuscular blocking drug-induced anaphylaxis. Anesthesiology
2011;115:657-9, author reply 659-60.
628. Baldo BA, Fisher MM, Pham NH. On the origin and specificity of antibodies
to neuromuscular blocking (muscle relaxant) drugs: an immunochemical
perspective. Clin Exp Allergy 2009;39:325-44.
629. Ebo DG, Fisher MM, Hagendorens MM, Bridts CH, Stevens WJ. Anaphylaxis
during anaesthesia: diagnostic approach. Allergy 2007;62:471- 87.
630. Florvaag E, Johansson SG, Oman H, Venemalm L, Degerbeck F, Dybendal T,
et al. Prevalence of IgE antibodies to morphine: relation to the high and low
incidences of NMBA anaphylaxis in Norway and Sweden, respectively. Acta
Anaesthesiol Scand 2005;49:437-44.
631. Florvaag E, Johansson SG, Oman H, Harboe T, Nopp A. Pholcodine stimu-
lates a dramatic increase of IgE in IgE-sensitized individuals: a pilot study.
Allergy 2006;61:49-55.
632. Harboe T, Johansson SG, Florvaag E, Oman H. Pholcodine exposure raises
serum IgE in patients with previous anaphylaxis to neuromuscular blocking
agents. Allergy 2007;62:1445-50.
633. Questions and answers on the review of the marketing authorisations for
medicines containing pholcodine. London, UK: European Medicines Agency.
2012. Available from: https://www.ema.europa.eu/en/documents/referral/
questions-answ ers-rev iew-mar keting-au thoris ations-medic ines-con taining-
pholcodine_en.pdf. Accessed July 28, 2020.
634. Seed MJ, Ewan PW. Anaphylaxis caused by neostigmine. Anaesthesia 2000;
55:574-5.
635. Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI,
et al. The diagnosis and management of anaphylaxis practice parameter: 2010
update. J Allergy Clin Immunol 2010;126:477-480.e1-e42.
636. Asserhoj LL, Mosbech H, Kroigaard M, Garvey LH. No evidence for con-
traindications to the use of propofol in adults allergic to egg, soy or peanut†.Br
J Anaesth 2016;116:77-82.
637. Belso N, Kui R, Szegesdi I, Kakuja M, Kapitany K, Kemeny L, et al.
Propofol and fentanyl induced perioperative anaphylaxis. Br J Anaesth
2011;106:283-4.
638. Cummings KC III, Arnaut K. Case report: fentanyl-associated intraoperative
anaphylaxis with pulmonary edema. Can J Anaesth 2007;54:301-6.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S110 BROYLES ET AL
639. De Pasquale TMA, Buonomo A, Pucci S. Delayed-type allergy to articaine
with cross-reactivity to other local anesthetics from the amide group. J Allergy
Clin Immunol Pract 2018;6:305-6.
640. Gonzalez-Delgado P, Anton R, Soriano V, Zapater P, Niveiro E. Cross-reac-
tivity among amide-type local anesthetics in a case of allergy to mepivacaine.
J Investig Allergol Clin Immunol 2006;16:311-3.
641. Venemalm L, Degerbeck F, Smith W. IgE-mediated reaction to mepivacaine.
J Allergy Clin Immunol 2008;121:1058-9.
642. Opstrup MS, Malling HJ, Kroigaard M, Mosbech H, Skov PS, Poulsen LK,
et al. Standardized testing with chlorhexidine in perioperative allergy–a large
single-centre evaluation. Allergy 2014;69:1390-6.
643. Tacquard C, Collange O, Gomis P, Malinovsky JM, Petitpain N, Demoly P, et al.
Anaesthetic hypersensitivity reactions in France between 2011 and 2012: the
10th GERAP epidemiologic survey. Acta Anaesthesiol Scand 2017;61:290-9.
644. Velicky P, Windsperger K, Petroczi K, Pils S, Reiter B , Weiss T, et al.
Pregnancy-associated diamine oxidase originates from extravillous tro-
phoblasts and is decreased in ea rly-onset pre eclampsia . Sci Rep 2018;8:
6342.
645. Dewachter P, Chollet-Martin S, Mouton-Faivre C, de Chaisemartin L, Nicaise-
Roland P. Comparison of basophil activation test and skin testing perfor-
mances in NMBA allergy. J Allergy Clin Immunol Pract 2018;6:1681-9.
646. Sadleir PH, Clarke RC, Platt PR. Cefalotin as antimicrobial prophylaxis in
patients with known intraoperative anaphylaxis to cefazolin. Br J Anaesth
2016;117:464-9.
647. Gueant JL, Mata E, Monin B, Moneret-Vautrin DA, Kamel L, Nicolas JP, et al.
Evaluation of a new reactive solid phase for radioimmunoassay of serum
specific IgE against muscle relaxa nt drugs. Allergy 1991;46:452-8.
648. Guilloux L, Ricard-Blum S, Ville G, Motin J. A new radioimmunoassay using
a commercially available solid support for the detection of IgE antibodies
against muscle relaxants. J Allergy Clin Immunol 1992;90:153-9.
649. Fisher MM, Baldo BA. Immunoassays in the diagnosis of anaphylaxis to
neuromuscular blocking drugs: the value of morphine for the detection of IgE
antibodies in allergic subjects. Anaesth Intensive Care 2000;28:167-70.
650. Kelly KJ, Sussman G. Latex allergy: where are we now and how did we get
there? J Allergy Clin Immunol Pract 2017;5:1212-6.
651. Mayorga C, Celik G, Rouzaire P, Whitaker P, Bonadonna P, Rodrigues-
Cernadas J, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/
EAACI Drug Allergy Interest Group position paper. Allergy 2016;71:1103-34.
652. Lafuente A, Javaloyes G, Berroa F, Goikoetxea MJ, Moncada R, Nunez-
Cordoba JM, et al. Early skin testing is effective for diagnosis of hypersen-
sitivity reactions occurring during anesthesia. Allergy 2013;68:820-2.
653. Miller J, Clough SB, Pollard RC, Misbah SA. Outcome of repeat anaesthesia
after investigation for perioperative anaphylaxis. Br J Anaesth 2018;120:
1195-201.
654. Schatz M. Skin testing and incremental challenge in the evaluation of adverse
reactions to local anesthetics. J Allergy Clin Immunol 1984;74:606-16.
655. Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician
2008;11:S133-53.
656. Levy JH, Rockoff MA. Anaphylaxis to meperidine. Anesth Analg 1982;61:
301-3.
657. Yoo HS, Yang EM, Kim MA, Hwang SH, Shin YS, Ye YM, et al. A case of
codeine induced anaphylaxis via oral route. Allergy Asthma Immunol Res
2014;6:95-7.
658. Zucker-Pinchoff B, Ramanathan S. Anaphylactic reaction to epidural fentanyl.
Anesthesiology 1989;71:599-601.
659. Kyrklund C, Hyry H, Alanko K. Allergic contact dermatitis caused by trans-
dermal buprenorphine. Contact Dermatitis 2013;69:60-1 .
660. Vander Hulst K, Parera Amer E, Jacobs C, Dewulf V, Baeck M, Pujol
Vallverdu RM, et al. Allergic contact dermatitis from transdermal buprenor-
phine. Contact Dermatitis 2008;59:366-9.
661. Nasser SM, Ewan PW. Opiate-sensitivity: clinical characteristics and the role
of skin prick testing. Clin Exp Allergy 2001;31:1014-20.
662. Flacke JW, Flacke WE, Bloor BC, Van Etten AP, Kripke BJ. Histamine
release by four narcotics: a double-blind study in humans. Anesth Analg 1987;
66:723-30.
663. Stutius LM, Pessach I, Lee J, Lo MS, Levy S, Schram P, et al. Sublingual
desensitization for buprenorphine hypersensitivity. J Allergy Clin Immunol
2010;125:938-9.
664. Gomes E, Cardoso MF, Praca F, Gomes L, Marino E, Demoly P. Self-reported
drug allergy in a general adult Portuguese population. Clin Exp Allergy 2004;
34:1597-601.
665. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin
induced asthma and its implications for clinical practice. BMJ 2004;328:434.
666. Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-
exacerbated respiratory disease among asthmatic patients: a meta-analysis of
the literature. J Allergy Clin Immunol 2015;135:676-81.e1.
667. Settipane GA. Aspirin and allergic diseases: a review. Am J Med 1983;74:
102-9.
668. Stevenson DD, Sanchez-Borges M, Szczeklik A. Classificat
ion of allergic and
pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann
Allergy Asthma Immunol 2001;87:177-80.
669. Kidon M, Blanca-Lopez N, Gomes E, Terreehorst I, Tanno L, Ponvert C, et al.
EAACI/ENDA Position Paper: diagnosis and management of hypersensitivity
reactions to non-steroidal anti-inflammatory drugs (NSAIDs) in children and
adolescents. Pediatr Allergy Immunol 2018;29:469-80.
670. Tuttle KL, Schneider TR, Henrickson SE, Morris D, Abonia JP, Spergel JM,
et al. Aspirin-exacerbated respiratory disease: not always “adult-onset”.
J Allergy Clin Immunol Pract 2016;4:756-8.
671. Dursun AB, Woessner KA, Simon RA, Karasoy D, Stevenson DD. Predicting
outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and
chronic sinusitis. Ann Allergy Asthma Immunol 2008;100:420-5.
672. Zisa G, Riccobono F, Bommarito L, D’Antonio C, Calamari AM, Poppa M,
et al. Provocation tests with the offending nonsteroidal anti-inflammatory
drugs in patients with urticaria/angioedema reactions. Allergy Asthma Proc
2012;33:421-6.
673. Himly M, Jahn-Schmid B, Pittertschatscher K, Bohle B, Grubmayr K,
Ferreira F, et al. IgE-mediated immediate-type hypersensitivity to the pyr-
azolone drug propyphenazone. J Allergy Clin Immunol 2003;111:882-8.
674. Weberschock TB, Muller SM, Boehncke S, Boehncke WH. Tolerance to
coxibs in patients with intolerance to non-steroidal anti-inflammatory drugs
(NSAIDs): a systematic structured review of the literature. Arch Dermatol Res
2007;299:169-75.
675. Quiralte J, Blanco C, Castillo R, Delgado J, Carrillo T. Intolerance to
nonsteroidal antiinflammatory drugs: results of controlled drug challenges in
98 patients. J Allergy Clin Immunol 1996;98:678-85.
676. Buchheit KM, Laidlaw TM. Update on the management of aspirin-exacerbated
respiratory disease. Allergy Asthma Immunol Res 2016;8:298-304.
677. White AA, Steve nson DD, Simon RA. The blockin g effect of essential
controller medications during aspirin challenges in patients with aspirin-
exacerbated resp iratory d isease. Ann A llergy Asth ma Immun ol 2005;95:
330-5.
678. Lee RU, Stevenson DD. Aspirin-exacerbated respiratory disease: evaluation
and management. Allergy Asthma Immunol Res 2011;3:3-10.
679. Chen JR, Buchmiller BL, Khan DA. An hourly dose-escalation desensitization
protocol for aspirin-exacerbated respiratory disease. J Allergy Clin Immunol
Pract 2015;3:926-31.e1.
680. Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use
of intranasal ketorolac and modified oral aspir in challenge for desensitization
of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol
2010;105:130-5.
681. Walters KM, Woessner KM. An overview of nonsteroidal antiinflammatory
drug reactions. Immunol Allergy Clin North Am 2016;36:625-41.
682. Cook KA, White AA. Rapid aspirin challenge in patients with aspirin allergy
and acute coronary syndromes. Curr Allergy Asthma Rep 2016;16:11.
683. Kvedariene V, Bencherioua AM, Messaad D, Godard P, Bousquet J,
Demoly P. The accuracy of the diagnosis of suspected paracetamol (acet-
aminophen) hypersensitivity: results of a single-blinded trial. Clin Exp Allergy
2002;32:1366-9.
684. Boussetta K, Ponvert C, Karila C, Bourgeois ML, Blic J, Scheinmann P.
Hypersensitivity reactions to paracetamol in children: a study of 25 cases.
Allergy 2005;60:1174-7.
685. Galindo PA, Borja J, Mur P, Feo F, Gomez E, Garcia R. Anaphylaxis to
paracetamol. Allergol Immunopathol (Madr) 1998;26:199-200.
686. Ho MH, Tung JY, Lee TL, Tsoi NS, Lau YL. Anaphylaxis to paracetamol.
J Paediatr Child Health 2008;44:746-7.
687. Martin JA, Lazaro M, Cuevas M, Alvarez-Cuesta E. Paracetamol anaphylaxis.
Clin Exp Allergy 1993;23:534.
688. Numata T, Fukushi R, Ito T, Tsuboi R, Harada K. Acetaminophen anaphylaxis
diagnosed by skin prick test. Allergol Int 2016;65:490-1.
689. Settipane RA, Schrank PJ, Simon RA, Mathison DA, Christiansen SC,
Stevenson DD. Prev alence of cross-sensitivity with acetaminophen in aspirin-
sensitive asthmatic subjects. J Allergy Clin Immunol 1995;96:480-5.
690. Stricker BH, Meyboom RH, Lindquist M. Acute hypersensitivity reactions to
paracetamol. Br Med J (Clin Res Ed) 1985;291:938-9.
691. Szczekl
ik A. Analgesics, allergy and asthma. Br J Clin Pharmacol 1980;10:
401S-5.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S111
692. Tsujino Y, Okamoto N, Morita E. Acetaminophen-induced urticaria without
aspirin intolerance. J Dermatol 2007;34:224-6.
693. Yilmaz O, Ertoy Karagol IH, Bakirtas A, Topal E, Celik GE, Demirsoy MS,
et al. Challenge-proven nonsteroidal anti-inflammatory drug hypersensitivity in
children. Allergy 2013;68:1555-61.
694. Van Diem L, Grilliat JP. Anaphylactic shock induced by paracetamol. Eur J
Clin Pharmacol 1990;38:389-90.
695. Leung R, Plomley R, Czarny D. Paracetamol anaphylaxis. Clin Exp Allergy
1992;22:831-3.
696. de Paramo BJ, Gancedo SQ, Cuevas M, Camo IP, Martin JA, Cosmes EL.
Paracetamol (acetaminophen) hypersensitivity. Ann Allergy Asthma Immunol
2000;85:508-11.
697. Rojas-Perez-Ezquerra P, Sanchez-Morillas L, Gomez-Traseira C, Gonzalez-
Mendiola R, Alcorta Valle AR, Laguna-Martinez J. Selective hypersensitivity
reactions to acetaminophen: a 13-case series. J Allergy Clin Immunol Pract
2014;2:343-5.
698. Ban GY, Ahn SJ, Yoo HS, Park HS, Ye YM. Stevens-Johnson syndrome and
toxic epidermal necrolysis associated with acetaminophen use during viral
infections. Immune Netw 2016;16:256-60.
699. Gabrielli S, Langlois A, Ben-Shoshan M. Prevalence of hypersensitivity re-
actions in children associated with acetaminophen: a systematic review and
meta-analysis. Int Arch Allergy Immunol 2018;176:106-14.
700. Caprie Steering Committee. A randomised, blinded, trial of clopidogrel versus
aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering
Committee. Lancet 1996;348:1329-39.
701. Plavix (clopidogrel bisulfate) prescribing information. Bridgewater, NJ: Bristol-
Myers Squibb Co and Sanofi Pharmaceuticals; 2019. Available from: https://
packageinserts.bms.com/pi/pi_plavix.pdf. Accessed July 29, 2020.
702. Henke PK. Commentary. Prasugrel versus clopidogrel in patients with acute
coronary symptoms. Perspect Vasc Surg Endovasc Ther 2008;20:223-4.
703. Lokhandwala J, Best PJ, Henry Y, Berger PB. Alle rgic reactions to clopidogrel
and cross-reactivity to other agents. Curr Allergy Asthma Rep 2011;11:52-7.
704. Lokhandwala JO, Best PJ, Butterfield JH, Skelding KA, Scott T,
Blankenship JC, et al. Frequency of allergic or hematologic adverse reactions
to ticlopidine among patients with allergic or hematologic adverse reactions to
clopidogrel. Circ Cardiovasc Interv 2009;2:348-51.
705. Campbell KL, Cohn JR, Fischman DL, Walinsky P, Mallya R, Jaffrani W,
et al. Management of clopidogrel hypersensitivity without drug interruption.
Am J Cardiol 2011;107:812-6.
706. Cheema AN, Mohammad A, Hong T, Jakubovic HR, Parmar GS, Sharieff W,
et al. Characterization of clopidogrel hypersensitivity reactions and manage-
ment with oral steroids without clopidogrel discontinuation. J Am Coll Cardiol
2011;58:1445-54.
707. von Tiehl KF, Price MJ, Valencia R, Ludington KJ, Teirstein PS, Simon RA.
Clopidogrel desensitization after drug-eluting stent placement. J Am Coll
Cardiol 2007;50:2039-43.
708. Peppard SR, Held-Godgluck BM, Beddingfield R. Use of prasugrel in a patient
with clopidogrel hypersensitivity. Ann Pharmacother 2011;45:e54.
709. Barbaud A, Reichert-Penetrat S, Trechot P, Jacquin-Petit MA, Ehlinger A,
Noirez V, et al. The use of skin testing in the investigation of cutaneou s
adverse drug reactions. Br J Dermatol 1998;139:49-58.
710. Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell
involvement in drug- induced acute generalized exanthematous pustulosis.
J Clin Invest 2001;107:1433-41.
711. Cuerda Galindo E, Goday Bujan JJ, Garcia Silva JM, Martinez W, Verea
Hernando M, Fonseca E. Fixed drug eruption from piroxicam. J Eur Acad
Dermatol Venereol 2004;18:586-7.
712. Chopra P, Verma P, Klaustermeyer WB. Successful use of prasugrel, an
alternative antiplatelet agent, in a patient with clopidogrel allergy. Ann Allergy
Asthma Immunol 2011;107:541-2.
713. Kim SH, Park SD, Baek YS, Lee SY, Shin SH, Woo SI, et al. Prasugrel-
induced hypersensitivity skin reaction. Korean Circ J 2014;44:355-7.
714. van Werkum JW, Braber TL, Verheggen PW, Van Der Have-Roeffel SM.
Prasugrel as alternative treatment strategy in a case with a hypersensitivity
reaction to clopidogrel. Platelets 2011;22:77-8.
715. Chin N, Rangamuwa K, Mariasoosai R, Carnes J, Thien F. Oral antiplatelet
agent hypersensitivity and cross-reactivity managed by successful desensiti-
sation. Asia Pac Allergy 2015;5:51-4.
716. Harris JR, Coons JC. Ticagrelor use in a patient with a documented clopidogrel
hypersensitivity. Ann Pharmacother 2014;48:1230-3.
717. Manchette AM, Drucker AG, Januzzi JL Jr. Acute coronary syndrome
antiplatelet alternatives in clopidogrel allergy. Pharmacotherapy 2014;34:
e152-e156.
718. Owen P, Garner J, Hergott L, Page RL II. Clopidogrel desensitization: case
report and review of published protocols. Pharmacotherapy 2008;28:259-70.
719. Fajt
M, Petrov A. Clopidogrel hypersensitivity: a novel multi-day outpatient
oral desensitization regimen. Ann Pharmacother 2010;44:11-8.
720. Vishnevsky A, Savage MP, Fischman DL. Treatment of clopidogrel hyper-
sensitivity: the Jefferson approach. Curr Vasc Pharmacol 2019;17:123-6.
721. Ramotowski B, Budaj A. Clopidogrel allergy successfully treated with corti-
costeroids without clopidogrel withdrawal. Kardiol Pol 2016;74:489.
722. Gonzalez-Delgado P, Fernandez J. Hypersensitivity reactions to heparins. Curr
Opin Allergy Clin Immunol 2016;16:315-22.
723. Koch P, Münssinger T, Rupp-John C, Uhl K. Delayed-type hypersensitivity
skin reactions caused by subcutaneous unfractionated and low-molecular-
weight heparins: tolerance of a new recombinant hirudin. J Am Acad Dermatol
2000;42:612-9.
724. Pföhler C, Müller CS, Pindur G, Eichler H, Schäfers HJ, Grundmann U, et al.
Delayed-type heparin allergy: diagnostic procedures and treatment alterna-
tives—a case series including 15 patients. World Allergy Organ J 2008;1:
194-9.
725. Anders D, Trautmann A. Allergic anaphylaxis due to subcutaneously injected
heparin. Allergy Asthma Clin Immunol 2013;9:1.
726. Phan C, Vial-Dupuy A, Autegarden JE, Amsler E, Gaouar H, Abuaf N, et al.
A study of 19 cases of allergy to heparins with positive skin testing. Ann
Dermatol Venereol 2014;141:23-9.
727. Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S,
Pelzer K, et al. Contaminated heparin associated with adverse clinical events
and activation of the contact system. N Engl J Med 2008;358:2457-67.
728. Gottschlich GM, Georgitis JW. Protamine-specific IgE, IgG, AND IgG sub-
class antibodies in protamine anaphylaxis. J Allergy Clin Immunol 1988;8 1:
238.
729. Porsche R, Brenner ZR. Allergy to protamine sulfate. Heart Lung 1999;28:
418-28.
730. Bircher AJ, Harr T, Hohenstein L, Tsakiris DA. Hypersensitivity reactions to
anticoagulant drugs: diagnosis and management options. Allergy 2006;61:
1432-40.
731. Vincent GM, Janowski M, Menlove R. Protamine allergy reactions during
cardiac catheterization and cardiac surgery: risk in patients taking protamine-
insulin preparations. Cathet Cardiovasc Diagn 1991;23:164-8.
732. Comunale ME, Maslow A, Robertson LK, Haering JM, Mashikian JS,
Lowenstein E. Effect of site of venous protamine administration, previously
alleged risk factors, and preoperative use of aspirin on acute protamine-induced
pulmonary vasoconstriction. J Cardiothor Vasc Anesth 2003;17:309-13.
733. Levy JH, Schwieger IM, Zaidan JR, Faraj BA, Weintraub WS. Evaluation of
patients at risk for protamine reacti ons. J Thoracic Cardiovasc Surg 1989;98:
200-4.
734. Ellis ER. Successful use of bivalirudin in place of heparin infusion for pul-
monary vein isolation using a cryoballoon catheter in a patient with heparin
allergy. HeartRhythm Case Rep 2017;3:10-2.
735. Jappe U, Gollnick H. Allergy to heparin, heparinoids, and recombinant hir-
udin: diagnostic and therapeutic alternatives. Hautarzt 1999;50:406-11.
736. Wütschert R, Piletta P, Bounameaux H. Adverse skin reactions to low mo-
lecular weight heparins: frequency, management and prevention. Drug Saf
1999;20:515-25.
737. Bottio T, Pittarello G, Bonato R, Fagiolo U, Gerosa G. Life-threatening
anaphylactic shock caused by porcine heparin intravenous infusion during
mitral valve repair. J Thorac Cardiovasc Surg 2003;126:1194-5.
738. Favaloro EJ, McCaughan G, Pasalic L. Clinical and laboratory diagnosis of
heparin induced thrombocytopenia: an update. Pathology 2017;49:346-55.
739. Keeling D, Davidson S, Watson H. Haemostasis and Thrombosis Task Force
of the British Committee for Standards in Haematology. The management of
heparin-induced thrombocytopenia. Br J Haematol 2006;133:259-69.
740. Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition,
treatment, and prevention: the Seventh ACCP Conference on Antithrombotic
and Thrombolytic Therapy. Chest 2004;126:311S-7S.
741. Morel DR, Costabella PM, Pittet JF. Adverse cardiopulmonary effects and
increased plasma thromboxane concentrations following the neutralization of
heparin with protamine in awake sheep are infusion rate-dependent. Anes-
thesiology 1990;73:415-24.
742. Raap U, Liekenbröcker T, Kapp A, Wedi B. Delayed-type hypersensitivity to
protamine as a complication of insulin therapy. Contact Dermatitis 2005;53:57-8.
743. Wu W, Cheng H, Bu R. Protamine-containing insulin allergy and renal
dysfunction in a patient with type 2 diabetes. J Diabetes Investig 2015;6:591-3.
744. Sullivan T. Drug allergy. In: Middleton Jr E, editor. Allergy: Principles and
Practice. St Louis: Mosby; 1993.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S112 BROYLES ET AL
745. Kim R. Anaphylaxis to protamine masquerading as an insulin allergy. Del Med
J 1993;65:17-23.
746. al-Eryani AY, al-Momen AK, Fayed DF, Allam AK. Successful heparin
desensitization after heparin-induced anaphylactic shock. Thrombosis Res
1995;79:523-6.
747. Dave S, Park MA. Successful heparin desensitization: a case report and review
of the literature. J Cardiac Surg 2008;23:394-7.
748. Kavut AB, Koca E. Successful desensitization with un-fractionated heparin in
a patient with heparin allergy and tolerance to fondaparinux. Asian Pac J
Allergy Immunol 2012;30:162-6.
749. Parekh K, Burkhart HM, Hatab A, Ross A, Muller BA. Heparin allergy:
successful desensitization for cardiopulmonary bypass. J Thorac Cardiovasc
Surg 2005;130:1455-6.
750. Patriarca G, Rossi M, Schiavino D, Schinco G, Fais G, Varano C, et al. Rush
desensitization in heparin hypersensitivity: a case report. Allergy 1994;49:
292-4.
751. Sokolowska E, Kalaska B, Miklosz J, Mogielnicki A. The toxicology of
heparin reversal with protamine: past, present and future. Expert Opin Drug
Metab Toxicol 2016;12:897-909.
752. Bollinger ME, Hamilton RG, Wood RA. Protamin e allergy as a complication
of insulin hypersensitivity: a case report. J Allergy Clin Immunol 1999;104:
462-5.
753. Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative
review. Orphanet J Rare Dis 2012;7:24.
754. Lillicrap D. von Willebrand disease: advances in pathogenetic understanding,
diagnosis, and therapy. Blood 2013;122:3735-40.
755. Cugno M, Gualtierotti R, Tedeschi A, Meroni PL. Au toantibodies to coagu-
lation factors: from pathophysiology to diagnosis and therapy. Autoimmun
Rev 2014;13:40-8.
756. Franchini M, Lippi G, Montagnana M, Targher G, Zaffanello M, Salvagno GL,
et al. Anaphylaxis in patients with congenital bleeding disorders and inhibitors.
Blood Coagul Fibrinolysis 2009;20:225-9.
757. Warrier I, Ewenstein BM, Ko erper MA, Shapiro A, Key N, DiMichele D, et al.
Factor IX inhibitors and anaphylaxis in hemophilia B. Haemophilia 1997;3:
231-2.
758. Kadar JG, Schuster J, Hunzelmann N. IgE-mediated anaphylactic reaction to
purified and recombinant factor VIII in a patient with severe haemophilia A.
Haemophilia 2007;13:104-5.
759. Shopnick RI, Kazemi M, Brettler DB, Buckwalter C, Yang L, Bray G, et al.
Anaphylaxis after treatment with recombinant factor VIII. Transfusion 1996;
36:358-61.
760. Helmer RE III, Alperin JB, Yunginger JW, Grant JA. Anaphylactic reactions
following infusion of factor VIII in a patient with classic hemophilia. Am J
Med 1980;69:953-7.
761. James PD, Lillicrap D, Mannucci PM. Alloantibodies in von Willebrand dis-
ease. Blood 2013;122:636-40.
762. Platt CD, D’Angelo L, Neufeld EJ, Broyles AD. Skin testing, graded chal-
lenge, and desensitization to von Willebrand factor (VWF) products in type III
von Willebrand disease (VWD). J Allergy Clin Immunol Pract 2016;4:1006-8.
763. Lak M, Peyvandi F, Mannucci PM. Clinical manifestations and complications
of childbirth and replacement therapy in 385 Iranian patients with type 3 von
Willebrand disease. Br J Haematol 2000;111:1236-9.
764. Dioun AF, Ewenstein BM, Geha RS, Schneider LC. IgE-mediated allergy and
desensitization to factor IX in hemophilia B. J Allergy Clin Immunol 1998;
102:113-7.
765. Jamieson DM, Stafford CT, Maloney MJ, Lutcher CL. Desensitization to
factor VIII in a patient with classic hemophilia and C2 deficiency. Ann Allergy
1987;58:215-20.
766. Franchini M, Veneri D, Lippi G. The use of recombinant activated factor VII in
congenital and acquired von Willebrand disease. Blood Coagul Fibrinolysis
2006;17:615-9.
767. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia.
Blood 2014;124:3365-72.
768. Benson G, Auerswald G, Elezovic I, Lambert T, Ljung R, Morfini M, et al.
Immune tolerance induction in patients with severe hemophilia with inhibitors:
expert panel views and recommendations for clinical practice. Eur J Haematol
2012;88:371-9.
769. Brockow K. Immediate and delayed cutaneous reactions to radiocontrast me-
dia. Chem Immunol Allergy 2012;97:180-90.
770. Scherer K, Harr T, Bach S, Bircher AJ. The role of iodine in hypersensitivity
reactions to radio contrast media. Clin Exp Allergy 2010;40:468-75.
771. Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P,
et al. Skin testing in patients with hypersensitivity reactions to iodinated
contrast media—a
European multicenter study. Allergy 2009;64:234-41.
772. Lerondeau B, Trechot P, Waton J, Poreaux C, Luc A, Schmutz JL, et al.
Analysis of cross-reactivity among radiocontr ast media in 97 hypersensitivity
reactions. J Allergy Clin Immunol 2016;137:633-5.e4.
773. Caimmi S, Benyahia B, Suau D, Bousquet-Rouanet L, Caimmi D,
Bousquet PJ, et al. Clinical value of negative skin tests to iodinated contrast
media. Clin Exp Allergy 2010;40:805-10.
774. Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA,
Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic
review, and Grading of Recommendations, Assessment, Development and
Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020;145:1082-123.
775. Kamm GL, Hagmeyer KO. Allergic-type reactions to corticosteroids. Ann
Pharmacother 1999;33:451-60.
776. Patel A, Bahna SL. Immediate hypersensitivity reactions to corticosteroids.
Ann Allergy Asthma Immunol 2015;115:178-82.e3.
777. Baker A, Empson M, The R, Fitzharris P. Skin testing for immediate hyper-
sensitivity to corticosteroids: a case series and literature review. Clin Exp
Allergy 2015;45:669-76.
778. Asakawa H, Araki T, Imai I, Tsutsumi Y, Kawakami F. Skin tests of steroid
allergy. Allergy 1999;54:645-6.
779. Figueredo E, Cuesta-Herranz JI, De Las Heras M, Lluch-Bernal M,
Umpierrez A, Sastre J. Anaphylaxis to dexamethasone. Allergy 1997;52:877.
780. Mace S, Vadas P, Pruzanski W. Anaphylactic shock induced by intraarticular
injection of methylprednisolone acetate. J Rheumatol 1997;24:1191-4.
781. Montoro J, Valero A, Serra-Baldrich E, Amat P, Lluch M, Malet A.
Anaphylaxis to paramethasone with tolerance to other corticosteroids. Allergy
2000;55:197-8.
782. Al Hadithy A, van Maaren M, Vermes A. Anaphylactic reactions following
Kenacort-A(R) injection: carboxymethylcellulose is involved once again.
Contact Dermatitis 2011;64:179-80.
783. Bordere A, Stockman A, Boone B, Franki AS, Coppens MJ, Lapeere H, et al.
A case of anaphylaxis caused by macrogol 3350 after injection of a cortico-
steroid. Contact Dermatitis 2012;67:376-8.
784. Caimmi S, Caimmi D, Bousquet PJ, Demoly P. Succinate as opposed to
glucocorticoid itself allergy. Allergy 2008;63:1641-3 .
785. Dewachter P, Mouton-Faivre C. Anaphylaxis to macrogol 4000 after a
parenteral corticoid injection. Allergy 2005;60:705-6.
786. Koutsostathis N, Vovolis V. Severe immunoglobulin E-mediated anaphylaxis
to intravenous methylprednisolone su ccinate in a patient who tolerated oral
methylprednisolone. J Investig Allergol Clin Immunol 2009;19:330-2.
787. Levy Y, Segal N, Nahum A, Marcus N, Garty BZ. Hypersensitivity to
methylprednisolone sodium succinate in children with milk allergy. J Allergy
Clin Immunol Pract 2014;2:471-4.
788. Matura M, Goossens A. Contact allergy to corticosteroids . Allergy 2000;55:
698-704.
789. Nowak-Wegrzyn A, Shapiro GG, Beyer K, Bardina L, Sampson HA.
Contamination of dry powder inhalers for asthma with milk proteins con-
taining lactose. J Allergy Clin Immunol 2004;113:558-60.
790. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis
induced by the carboxymethylcellulose component of injectable triamcinolone
acetonide suspension (Kenalog). Ann Allergy Asthma Immunol 1995;74:163-6.
791. Savvatianos S, Giavi S, Stefanaki E, Siragakis G, Manousakis E,
Papadopoulos NG. Cow’s milk allergy as a cause of anaphylaxis to systemic
corticosteroids. Allergy 2011;66:983-5.
792. Sohy C, Vandenplas O, Sibille Y. Usefulness of oral macrogol challenge in
anaphylaxis after intra-articular injection of corticosteroid preparation. Allergy
2008;63:478-9.
793. Spoerl D, Scherer K, Bircher AJ. Contact urticaria with systemic symptoms
due to hexylene glycol in a topical corticosteroid: case report and review of
hypersensitivity to glycols. Dermatology 2010;220:238-42.
794. Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical
corticosteroids, is an important contact allergen. Dermatitis 2008;19:323-7.
795. Baeck M, Chemelle JA, Terreux R, Drieghe J, Goossens A. Delayed hyper-
sensitivity to corticosteroids in a series of 315 patients: clinical data and patch
test results. Contact Dermatitis 2009;61:163-75.
796. Boffa MJ, Wilkinson SM, Beck MH. Screening for corticosteroid contact
hypersensitivity. Contact Dermatitis 1995;33:149-51.
797. Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma
Immunol 2014;113:9-12.
798. Hogan D, Ledet JJ. Impact of regulation on contact dermatitis. Dermatol Clin
2009;27:385-94. viii.
799. Wolf R, Orion E, Ruocco E, Baroni A, Ruocco V. Contact dermatitis: facts and
controversies. Clin Dermatol 2013;31:467-78.
800. Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by pre-
servatives. Dermatitis 2014;25:215-31.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S113
801. Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction
patterns in allergic contact dermatitis fro m topical corticosteroids. Br J Der-
matol 1989;121:27-34.
802. Rachid R, Leslie D, Schneider L, Twarog F. Hypersensitivity to systemic
corticosteroids: an infrequent but potentially life-threatening condition.
J Allergy Clin Immunol 2011;127:524-8.
803. Rodrigues-Alves R, Spinola-Santos A, Pedro E, Branco-Ferreira M, Pereira-
Barbosa M. Immediate hypersensitivity to corticosteroids: finding an alterna-
tive. J Investig Allergol Clin Immunol 2007;17:284-5.
804. Venturini M, Lobera T, del Pozo MD, Gonzalez I, Blasco A. Immediate hy-
persensitivity to corticosteroids. J Investig Allergol Clin Immunol 2006;16:51-6.
805. Calogiuri GF, Muratore L, Nettis E, Ventura MT, Ferrannini A, Tursi A.
Anaphylaxis to hydrocortisone hemisuccinate with cross-sensitivity to related
compounds in a paediatric patient. Br J Dermatol 2004;151:707-8.
806. Ventura MT, Calogiuri GF, Matino MG, Dagnello M, Buquicchio R, Foti C,
et al. Alternative glucocorticoids for use in cases of adverse reaction to sys-
temic glucocorticoids: a study on 10 patients. Br J Dermatol 2003;148:139-41.
807. Angel-Pereira D, Berges-Gimeno MP, Madrigal-Burgaleta R, Urena-
Tavera MA, Zamora-Verduga M, Alvarez-Cuesta E. Successful rapid desen-
sitization to methylprednisolone sodium hemisuccinate: a case report. J Allergy
Clin Immunol Pract 2014;2:346-8.
808. Currie GP, Paterson E, Keenan F, Nath S, Watt SJ. An unexpected response to
intravenous hydrocortisone succinate in an asthmatic patient. Br J Clin Phar-
macol 2005;60:342.
809. Gelincik A, Yazici H, Emre T, Yakar F, Buyukozturk S. An alternative
approach to a renal transplant patient who experienced an immediate type
systemic reaction due to methylprednisolone sodium succinate. J Investig
Allergol Clin Immunol 2009;19:162-3.
810. Nucera E, Lombardo C, Aruanno A, Colagiovanni A, Buonomo A, de
Pasquale T, et al. ‘Empty sella syndrome’: a case of a patient with sodium
succinate hydrocortisone allergy. Eur J Endocrinol 2011;164:139-40.
811. Walker AI, Rawer HC, Sieber W, Przybilla B. Immediate-type hypersensitivity
to succinylated corticosteroids. Int Arch Allergy Immunol 2011;155:86-92.
812. Eda A, Sugai K, Shioya H, Fujitsuka A, Ito S, Iwata T, et al. Acute allergic
reaction due to milk proteins contaminating lactose added to corticosteroid for
injection. Allergol Int 2009;58:137-9.
813. Field S, Falvey E, Barry J, Bourke J. Type 1 hypersensitivity reaction to
carboxymethylcellulose following intra-articular triamcinolone injection.
Contact Dermatitis 2009;61:302-3.
814. Laing ME, Fallis B, Murphy GM. Anaphylactic reaction to intralesional
corticosteroid injection. Contact Dermatitis 2007;57:132-3.
815. Moran DE, Moynagh MR, Alzanki M, Chan VO, Eustace SJ. Anaphylaxis at
image-guided epidural pain block secondary to corticosteroid compound.
Skeletal Radiol 2012;41:1317-8.
816. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis
to intraarticular glucocorticoid preparations. AJR Am J Roentgenol 2009;193:
W156-W157.
817. Coloe J, Zirwas MJ. Allergens in corticosteroid vehicles. Dermatitis 2008;19:
38-42.
818. Funk JO, Maibach HI. Propylene glycol dermatitis: re-evaluation of an old
problem. Contact Dermatitis 1994;31:236-41.
819. Warshaw EM, Botto NC, Maibach HI, Fowler JF Jr, Rietschel RL, Zug KA,
et al. Positive patch-test reactions to propylene glycol: a retrospective cross-
sectional analysis from the North American Contact Dermatitis Group, 1996 to
2006. Dermatitis 2009;20:14-20.
820. Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the
TRUE test. J Clin Aesthet Dermatol 2010;3:36-41.
821. Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact
Dermatitis 1997;36:114.
822. Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long-term
use of proton pump inhibitors. World J Gastroenterol 2010;16:2323-30.
823. Bose S, Guyer A, Long A, Banerji A. Evaluation and management of hypersen-
sitivity to protonpumpinhibitors.Ann AllergyAsthma Immunol2013;111:452-7.
824. Kepil Ozdemir S, Yilmaz I, Aydin O, Buyukozturk S, Gelincik A,
Demirturk M, et al. Immediate-type hypersensitivity reactions to proton pump
inhibitors: usefulness of skin tests in the diagnosis and assessment of cross-
reactivity. Allergy 2013;68:1008-14.
825. Sanchez-Morillas L, Rojas Perez-Ezquerra P, Gonzalez Mendiola R, Gomez-
Tembleque Ubeda P, Santos Alvarez A, Laguna-Martinez JJ. Eleven cases of
omeprazole hypersensitivity: diagnosis and study of cross-reactivity. J Investig
Allergol Clin Immunol 2014;24:130-2.
826. Lobera T, Navarro B, Del Pozo MD, Gonzalez I, Blasco A, Escudero R, et al.
Nine cases of omeprazole allergy: cross-reactivity between proton pump in-
hibitors. J Investig Allergol Clin Immunol 2009;19:57-60.
827. Mota I, Gaspar A, Chambel M, Morais-Almeida M. Anaphylaxis induced by
proton pump inhibitors. J Allergy Clin Immunol Pract 2016;4:535-6.
828. Sobrev
ia Elfau MT, Garces Sotillos M, Ferrer Claveria L, Segura Arazuri N,
Monzon Ballarin S, Colas Sanz C. Study of cross-reactivity between proton
pump inhibitors. J Investig Allergol Clin Immunol 2010;20:157-61.
829. Otani IM, Banerji A. Immediate and delayed hypersen sitivity reactions to
proton pump inhibitors: evaluation and management. Curr Allergy Asthma
Rep 2016;16:17.
830. Perez Pimiento AJ, Prieto Lastra L, Rodriguez Cabreros MI, Gonzalez
Sanchez LA, Mosquera MR, Cubero AG. Hypersensitivity to lansoprazole and
rabeprazole with tolerance to other proton pump inhibitors. J Allergy Clin
Immunol 2006;117:707-8.
831. Porcel S, Rodriguez A, Jimenez S, Alvarado M, Hernandez J. Allergy to
lansoprazole: study of cross-reactivity among proton-pump inhibitors. Allergy
2005;60:1087-8.
832. Bourneau-Martin D, Leclech C, Jamet A, Drablier G, Trenque T, Juengel K,
et al. Omeprazole-induced drug reaction with eosinophilia and systemic
symptoms (DRESS). Eur J Dermatol 2014;24:413-5.
833. Ghatan PH, Marcusson-Stahl M, Matura M, Bjorkheden C, Lundborg P,
Cederbrant K. Sensitization to omeprazole in the occupational setting. Contact
Dermatitis 2014;71:371-5.
834. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control
study indicates an increased risk of acute interstitial nephritis with proton pump
inhibitor use. Kidney Int 2014;86:837-44.
835. Sandholdt LH, Laurinaviciene R, Bygum A. Proton pump inhibitor-induced
subacute cutaneous lupus erythematosus. Br J Dermatol 2014;170:342-51.
836. Turedi O, Sozener ZC, Kendirlinan R, Bavbek S. A case of pantoprazole
anaphylaxis with cross reactivity to all proton pump inhibitors: finding a safe
alternative. Curr Drug Saf 2017;12:198-200.
837. Laguna JJ, Bogas G, Salas M, Mayorga C, Dionicio J, Gonzalez-Mendiola R,
et al. The basophil activation test can be of value for diagnosing immediate
allergic reactions to omeprazole. J Allergy Clin Immunol Pract 2018;6:
1628-1636.e2.
838. Benito-Garcia F, Chambel M, Morais-Almeida M. Anaphylaxis due to proton
pump inhibitors: current understanding and important clinical considerations.
Expert Rev Clin Immunol 2018;14:653-6.
839. Confino-Cohen R, Goldberg A. Anaphylaxis to omeprazole: diagnosis and
desensitization protocol. Ann Allergy Asthma Immunol 2006;96:33-6.
840. Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad
Dermatol 1979;1:365-74.
841. Excess of ampicillin rashes associated with allopurinol or hyperuricemia: a
report from the Boston Collaborative Drug Surveillance Program, Boston
University Medical Center. N Engl J Med 1972;286:505-7.
842. Ramasamy SN, Korb-Wells CS, Kannanga ra DR, Smith MW, Wang N,
Roberts DM, et al. Allopurinol hypersensitivity: a systematic review of all
published cases, 1950-2012. Drug Saf 2013;36:953-80.
843. Yun J, Mattsson J, Schnyder K, Fontana S, Largiader CR, Pichler WJ,
et al. Allopurinol hypersensitivity is primarily mediated by dose-depen-
dent oxypurinol-specific T cell response. Clin Exp Allergy 2013;43:
1246-55.
844. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-
B*5801 allele as a genetic marker for severe cutaneous adverse reactions
caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134-9.
845. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012
American College of Rheumat ology guidelines for management of gout, part
1: systematic nonpharmacologic and pharmacologic therapeutic approaches to
hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431-46.
846. Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous
reactions requiring hospitalization in allopurinol initiators: a population-based
cohort study. Arthritis Care Res (Hoboken) 2013;65:578-84.
847. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes
Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis:
assessment of medication risks with emphasis on recently marketed drugs. The
EuroSCAR-study. J Invest Dermatol 2008;128:35-44.
848. Gelbart DR, Weinstein AB, Fajardo LF. Allopurinol-induced interstitial
nephritis. Ann Intern Med 1977;86:196-8.
849. Magner P, Sweet J, Bear RA. Granulomatous interstitial nephritis associated
with allopurinol therapy. CMAJ 1986;135:496-7.
850. Walz-LeBlanc BA, Reynolds WJ, MacFadden DK. Allopurinol sensitivity in a
patient with chronic tophaceous gout: success of intravenous desensitization
after failure of oral desensitization. Arthritis Rheum 1991;34:1329-31.
851. Schumacher MJ, Copeland JG. Intravenous desensitization to allopurinol in a
heart transplant patient with gout. Ann Allergy Asthma Immunol 2004;92:
374-6.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S114 BROYLES ET AL
852. Toker O, Tvito A, Rowe JM, Ashkenazi J, Ganzel C, Tal Y, et al. Rapid oral
allopurinol desensitization in a patient with chronic myeloid leukemia. Isr Med
Assoc J 2014;16:461-2.
853. Dursun AB, Sahin OZ. Allopurinol desensitization with a 2 weeks modified
protocol in an elderly patients with multiple comorbidities: a case report. Al-
lergy Asthma Clin Immunol 2014;10:52.
854. Fam AG, Dunne SM, Iazzetta J, Paton TW. Efficacy and safety of desensiti-
zation to allopurinol following cutaneous reactions. Arthritis Rheum 2001;44:
231-8.
855. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syn-
drome: incidence, prevention and management. Drug Saf 1999;21:489-501.
856. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical
genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428:486.
857. Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic
symptoms: understanding the role of virus and HLA in severe cutaneous drug
allergy. J Allergy Clin Immunol Pract 2014;2 :21-33.
858. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D,
Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensi-
tivity reactions in Europeans. N Engl J Med 2011;364:1134-43.
859. Knudsen JF, Flowers CM, Kortepeter C, Awaad Y. Clinical pro fi le of oxcar-
bazepine-related angioneurotic edema: case report and review. Pediatr Neurol
2007;37:134-7.
860. Mondal R, Sarkar S, Sabui T, Pan PP. Phenytoin induced life threatening
macroglossia in a child. J Neurosci Rural Pract 2013;4:75-7.
861. Wang XQ, Shi XB, Au R, Chen FS, Wang F, Lang SY. Influence of chemical
structure on skin reactions induced by antiepileptic drugs–the role of the ar-
omatic ring. Epilepsy Res 2011;94:213-7.
862. Phillips EJ, Mallal SA. HLA-B*1502 screening and toxic effects of carba-
mazepine. N Engl J Med 2011;365:672, author reply 673.
863. Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-
induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med
2011;364:1126-33.
864. Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on
antiepileptic drug use and severe skin reactions. Neurology 2014;83:2077-84.
865. Amstutz U, Shear NH, Rieder MJ, Hwang S, Fung V, Nakamura H, et al.
Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to
reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia
2014;55:496-506.
866. Leckband SG, Kelsoe JR, Dunnenberger HM, George AL Jr, Tran E, Berger R,
et al. Clinical Pharmacogenetics Implementation Consortium guidel ines for
HLA-B genotype and carbamazepine dosing. Clin Pharmacol Therapeut 2013;
94:324-8.
867. Yip VL, Marson AG, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA ge-
notype and carbamazepine-induced cutaneous adverse drug reactions: a sys-
tematic review. Clin Pharmacol Therapeut 2012;92:757-65.
868. Phillips EJ, Sukasem C, Whirl-Carrillo M, Muller DJ, Dunnenb erger HM,
Chantratita W, et al. Clinical Pharmacogenetics Implementation Consortium
(CPIC) guideline for HLA genotype and use of carbamazepine and oxcarba-
zepine: 2017 update. Clin Pharmacol Therapeut 2018;103:574-81.
869. Patsalos PN, Froscher W, Pisani F, van Rijn CM. The importance of drug
interactions in epilepsy therapy. Epilepsia 2002;43:365-85.
870. Elzagallaai AA, Knowles SR, Rieder MJ, Bend JR, Shear NH, Koren G. Patch
testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a sys-
tematic review. Drug Saf 2009;32:391-408.
871. Lin YT, Chang YC, Hui RC, Yang CH, Ho HC, Hung SI, et al. A patch testing
and cross-sensitivity study of carbamazepine-induced severe cutaneous
adverse drug reactions. J Eur Acad Dermatol Venereol 2013;27:356-64.
872. Shiny TN, Mahajan VK, Mehta KS, Chauhan PS, Rawat R, Sharma R. Patch
testing and cross sensitivity study of adverse cutaneous drug reactions due to
anticonvulsants: a preliminary report. World J Methodol 2017;7:25-32.
873. Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, et al.
Clinical pharmacogenetics implementation consortium guidelines for CYP2C9
and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Therapeut 2014;
96:542-8.
874. Chung WH, Hung SI. Genetic factors associated with severe cutaneous adverse
reactions–reply. JAMA 2014;312:2166.
875. Wang W, Hu FY, Wu XT, An DM, Yan B, Zhou D. Genetic susceptibility to
the cross-reactivity of aromatic antiepileptic drugs-induced cutaneous adverse
reactions. Epilepsy Res 2014;108:1041-5.
876. Elzagallaai AA, Knowles SR, Rieder MJ, Bend JR, Shear NH, Koren G. In
vitro testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a
systematic review. Mol Diagnosis Ther 2009;13:313-30.
877. Butte
MJ, Dodson B, Dioun A. Pentobarbital desensitization in a 3-month-old
child. Allergy Asthma Proc 2004;25:225-7.
878. Lee B, Yu HJ, Kang ES, Lee M, Lee J. Human leukocyte antigen genotypes
and trial of desensitization in patients with oxcarbazepine-induced skin rash: a
pilot study. Pediatr Neurol 2014;51:207-14.
879. Lee J, Park EG, Lee M, Lee J. Desensitization to oxcarbazepine: long-term
efficacy and tolerability. J Clin Neurol 2017;13:47-54.
880. Froscher W, Kleinhans D. Succe ssful induction of tolerance in an epilepsy
patient with phenobarbital allergy. Nervenarzt 1998;69:158-61.
881. Toker O, Tal Y, Horev L, Shmoeli D, Gilboa T. Valproic acid hypersensitivity
and desensitization. Dev Med Child Neurol 2015;57:1076-8.
882. Asero R. Hypersensitivity to diazepam. Allergy 2002;57:1209.
883. Barbaud A, Girault PY, Schmutz JL, Weber-Muller F, Trechot P. No cross-
reactions between tetrazepam and other benzodiazepines: a possible chemical
explanation. Contact Dermatitis 2009;61:53-6.
884. Kampgen E, Burger T, Brocker EB, Klein CE. Cross-reactive type IV hy-
persensitivity reactions to benzodiazepines revealed by patch testing. Contact
Dermatitis 1995;33:356-7.
885. Martinez-Tadeo JA, Perez- Rodriguez E, Hernandez-Santana G, Garcia-
Robaina JC, de la Torre-Morin F. Anaphylaxis caused by tetrazepam without
cross-reactivity with other benzodiazepines. Ann Allergy Asthma Immunol
2012;108:284-5.
886. Martin-Merino E, de Abajo FJ, Gil M. Risk of toxic epidermal necrolysis and
Stevens-Johnson syndrome associated with benzodiazepines: a population-
based cohort study. Eur J Clin Pharmacol 2015;71:759-66.
887. Shrivastava S. An experience with midazolam anaphylactoid reaction. J Anesth
2012;26:642-3.
888. Hagau N, Bologa RO, Indrei CL, Longrois D, Dirzu DS, Gherman-Ionica N.
Maximum non-reactive concentration of midazolam and ketamine for skin testing
study in non-allergic healthy volunteers. Anaesth Intensive Care 2010;38:513-8.
889. Swinnen I, Ghys K, Kerre S, Constandt L, Goossens A. Occupational airborne
contact dermatitis from benzodiazepines and other drugs. Contact Dermatitis
2014;70:227-32.
890. Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al.
Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.
1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthal-
mology 2012;119:347-54.
891. Lonnerholm G, Widerlov E. Effect of intravenous atropine and methylatropine
on heart rate and secretion of saliva in man. Eur J Clin Pharmacol 1975;8:
233-40.
892. Cabrera-Freitag P, Gastaminza G, Goikoetxea MJ, Lafuente A, de la
Borbolla JM, Sanz ML. Immediate allergic reaction to atropine in ophthalmic
solution confirmed by basophil activation test. Allergy 2009;64:1388-9.
893. Coelho D, Fernandes T, Branga P, Malheiro D, Rodrigues J. Intraoperative
anaphylaxis after intravenous atropine. Eur J Anaesthesiol 2007;24:289-90.
894. Tayman C, Mete E, Catal F, Akca H. Anaphylactic reaction due to cyclo -
pentolate in a 4-year-old child. J Investig Allergol Clin Immunol 2010;20:
347-8.
895. Aguilera L, Martinez-Bourio R, Cid C, Arin o JJ, Saez de Eguilaz JL,
Arizaga A. Anaphylactic reaction after atropine. Anaesthesia 1988;43:955-7.
896. Pemberton MN, Yar R, Sloan P. Fixed drug eruption to oxybutynin. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod 2008;106:e19-e21.
897. Issak A, Nystrom P. Cutaneous drug eruption associated with all FDA
approved inhaled muscarinic antagonists. Am J Respir Crit Care Med 2015;
191:A5646.
898. Cavanah DK, Casale TB. Cutaneous responses to anticholinergics: evidence
for muscarinic receptor subtype participation. J Allergy Clin Immunol 1991;
87:971-6.
899. Fisher MM, Bowey CJ. Intradermal compared with prick testing in the diag-
nosis of anaesthetic allergy. Br J Anaesth 1997;79:59-63.
900. Szebeni J, Fishbane S, Hedenus M, Howaldt S, Locatelli F, Patni S, et al.
Hypersensitivity to intravenous iron: classification, terminology, mechanisms
and management. Br J Pharmacol 2015;172:5025-36.
901. Tovbin D, Mazor D, Vorobiov M, Chaimovitz C, Meyerstein N. Induction of
protein oxidation by intravenous iron in hemodialysis patients: role of
inflammation. Am J Kidney Dis 2002;40:1005-12.
902. Coyne DW, Adkinson NF, Nissenson AR, Fishbane S, Agarwal R,
Eschbach JW, et al. Sodium ferric gluconate complex in hemodialysis patients,
II: adverse reactions in iron dextran-sensitive and dextran-tolerant patients.
Kidney Int 2003;63:217-24.
903. Santosh S, Podaralla P, Miller B. Anaphylaxis with elevated serum tryptase
after administration of intravenous ferumoxytol. NDT Plus 2010;3:341-2.
J ALLERGY CLIN IMMUNOL PRACT
VOLUME 8, NUMBER 9S
BROYLES ET AL S115
904. Barton JC, Barton EH, Bertoli LF, Gothard CH, Sherrer JS. Intravenous iron
dextran therapy in patients with iron deficiency and normal renal function who
failed to respond to or did not tolerate oral iron supplementation. Am J Med
2000;109:27-32.
905. Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The
safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis
1996;28:529-34.
906. Wysowski DK, Swartz L, Borders-Hemphill BV, Goulding MR, Dormitzer C.
Use of parenteral iron products and serious anaphylactic-type reactions. Am J
Hematol 2010;85:650-4.
907. Chandler G, Harchowal J, Macdougall IC. Intravenous iron sucrose: estab-
lishing a safe dose. Am J Kidney Dis 2001;38:988-91.
908. Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug
events associated with parenteral iron. Nephrol Dialysis Transplant 2006;21:
378-82.
909. Fletes R, Lazarus JM, Gage J, Chertow GM. Suspected iron dextran-related
adverse drug events in hemodialysis patients. Am J Kidney Dis 2001;37:
743-9.
910. McCarthy JT, Regnier CE, Loebertmann CL, Bergstral h EJ. Adverse events in
chronic hemodialysis patients receiving intravenous iron dextran–a comparison
of two products. Am J Nephrol 2000;20:455-62.
911. Mulder MB, van den Hoek HL, Birnie E, van Tilburg AJP,
Westerman EM. Westerman EM. Comparison of hypersensitivity reactions
of intravenous iron: iron isomaltoside-1000 (Monofer
Ò
) versus ferric car-
boxy-maltose (Ferinject
Ò
). A single center, cohort study. Br J Clin Phar-
macol 2019;85:385-92.
912. Christoph P, Schuller C, Studer H, Irion O, De Tejada BM, Surbek D. Intra-
venous iron treatment in pregnancy: comparison of high-dose ferric carbox-
ymaltose vs. iron sucrose. J Perin Med 2012;40:469-74.
913. Mahey R, Kriplani A, Mogili KD, Bhatla N, Kachhawa G, Saxena R. Ran-
domized controlled trial comparing ferric carboxymaltose and iron sucrose for
treatment of iron deficiency anemia due to abnormal uterine bleeding. Int J
Gynaecol Obstetr 2016;133:43-8.
914. Okam MM, Mandell E, Hevelone N, Wentz R, Ross A, Abel GA. Comparative
rates of adverse events with different formulations of intravenous iron. Am J
Hematol 2012;87:E123-E124.
915. Pfenniger A, Schuller C, Christoph P, Surbek D. Safety and efficacy of high-
dose intravenous iron carboxymaltose vs. iron sucrose for treatment of post-
partum anemia. J Perinat Med 2012;40:397-402.
916. Rampton D, Folkersen J, Fishbane S, Hedenus M, Howaldt S, Locatelli F,
et al. Hypersensitivity reactions to intravenous iron: guidance for risk mini-
mization and management. Haematologica 2014;99:1671-6.
917. Sav T, Tokgoz B, Sipahioglu MH, Deveci M, Sari I, Oymak O, et al. Is there a
difference between the allergic potencies of the iron sucrose and low molecular
weight iron dextran? Renal Failure 2007;29:423-6.
918. Adkinson NF, Strauss WE, Macdougall IC, Bernard KE, Auerbach M, Kaper RF,
et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose
in iron deficiency anemia: a randomized trial. Am J Hematol 2018;93:683-90.
919. Bailie GR. Comparison of rates of reported adverse events associated with i.v.
iron products in the United States. Am J Health-Syst Pharm 2012;69:310-20.
920. Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: ferumoxytol for intra-
venous iron therapy in adult patients with chronic kidney disease. Am J
Hematol 2010;85:315-9.
921. Macdougall IC, Strauss WE, McLaughlin J, Li Z, Dellanna F, Hertel J.
A randomized comparison of ferumoxytol and iron sucrose for treating iron
deficiency anemia in patients with CKD. Clin J Am Soc Nephrol 2014;9:705-12.
922. Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA,
Stenvinkel P, et al. Iron management in chronic kidn ey disease: conclusions
from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Contro-
versies Conference. Kidney Int 2016;89:28-39.
923. Bastani B, Rahman S, Gellens M. Lack of reaction to ferric gluconate in he-
modialysis patients with a history of severe reaction to iron dextran. ASAIO J
2002;48:404-6.
924. Van Wyck DB, Cavallo G, Spinowitz BS, Adhikarla R, Gagnon S,
Charytan C, et al. Safety and efficacy of iron sucrose in patients sensitive to
iron dextran: North American clinical trial. Am J Kidney Dis 2000;36:88-97.
925. Altman LC, Petersen PE. Successful prevention of an anaphylactoid reaction to
iron dextran. Ann Intern Med 1988;109:346-7.
926. Monaghan MS, Glasco G, St John G, Bradsher RW, Olsen KM. Safe
administration of iron dextran to a patient who reacted to the test dose.
Southern Med J 1994;87:1010-2.
927. Jimenez B, Dominguez-Ortega J, Nunez-Acevedo B, Cava-Sumner B, Kin-
delan-Recarte C, Montojo-Guillen C. Rapid iron desensitization after gener-
alized urticaria and facial angioedema. Investig Allergol Clin Immunol 2014;
24:69-71.
928. Rodriguez-Jimenez B, Dominguez-Ortega J, Nunez-Acevedo B, Cava-
Sumner B, Kindelan-Recarte C, Mon tojo-Guillen C. Rapid iron desensitization
after generalized urticaria and facial angioedema. J Investig Allergol Clin
Immunol 2014;24:69-71.
929. Romano A, Di Fonso M, Viola M, Adesi FB, Venuti A. Selective hypersen-
sitivity to piperacillin. Allergy 2000;55:787.
930. Romano A, Gueant-Rodriguez RM, Viola M, Amoghly F, Gaeta F, Nicolas JP,
et al. Diagnosing immediate reactions to cephalosporins. Clin Exp Allergy
2005;35:1234-42.
931. Gaig P. Selective type-1 hypersensitivity to cefixime. Allergy 1999;54:901-2.
932. Bonfanti P, Pusterla L, Parazzini F, Libanore M, Cagni AE, Franzetti M, et al.
The effectiveness of desensitization versus rechallenge treatment in HIV-
positive patients with previous hypersensitivity to TMP-SMX: a randomized
multicentric study. C.I.S.A.I. Group. Biomed Pharmacother 2000;54:45-9.
933. Lantner RR. Ciprofloxacin desensitization in a patient with cystic fibrosis.
J Allergy Clin Immunol 1995;96:1001-2.
934. Thomas M, Hopkins C, Duffy E, Lee D, Loulergue P, Ripamonti D, et al.
Association of the HLA-B*53:01 allele with drug reaction with eosinophilia
and
systemic symptoms (DRESS) syndrome during treatment of HIV infection
with raltegravir. Clin Infect Dis 2017;64:1198-203.
935. Heinzerling L, Raile K, Rochlitz H, Zuberbier T, Worm M. Insulin allergy: clinical
manifestations and management strategies. Allergy 2008;63:148-55.
936. Garon SL, Pavlos RK, White KD, Brown NJ, Stone CA Jr, Phillips EJ.
Pharmacogenomics of off-target adverse drug reactions. Br J Clin Pharmacol
2017;83:1896-911.
J ALLERGY CLIN IMMUNOL PRACT
OCTOBER 2020
S116 BROYLES ET AL
