
Abstract. Background/aim: The antiparasitic drug,
ivermectin (IVM), exerts anticancer activities in diverse
cancer types. However, its anticancer activity against
cholangiocarcinoma (CCA), especially the drug-resistant
phenotype, has not yet been explored. Materials and
Methods: IVM was tested for its anticancer activity against
gemcitabine-sensitive (KKU214) and gemcitabine-resistant
(KKU214
GemR
) CCA cell lines in vitro using the
sulforhodamine B and clonogenic assays as well as cell-
cycle analysis. Results: IVM treatment inhibited cell
proliferation and colony formation of both KKU214 and
KKU214
GemR
in a dose- and time-dependent manner.
KKU214
GemR
cells were more sensitive than KKU214 to
IVM treatment. IVM treatment caused S-phase cell-cycle
arrest and also cell death as indicated by an increase of sub-
G
0
/G
1
population in KKU214
GemR
cells treated with IVM for
48 h. Conclusion: IVM exerts anti-CCA activities and
gemcitabine-resistant KKU214
GemR
cells are more sensitive
to IVM treatment. Thus, IVM might be useful as an
alternative treatment for CCA, especially in patients who do
not respond to gemcitabine.
Chemotherapeutic drugs have been developed and used as
first-line treatment of several cancers. Although satisfactory
outcomes are usually achieved at the beginning of treatment,
the efficacy any drug tends to decline over time. Cancer cells
can adapt to the toxic effects of chemotherapy and eventually
become tolerant of the drug (1, 2). Drug resistance is the
major impediment to effective cancer therapy and has been
observed in several cancers including cholangiocarcinoma
(CCA), a bile-duct cancer originating from bile-duct
epithelial cells. Although surgical resection can be effective
for CCA treatment, approximately two-thirds of CCA
patients are inoperable (3). Hence, chemotherapy is a
treatment of choice and has been used to control disease
progression and improve survival and quality of life for
unresectable CCA patients (4). Gemcitabine (2’, 2’-
difluorodeoxycytidine) is a deoxycytidine analogue which
has been widely used for treatment of CCA (3). However,
several clinical studies have indicated that response rates and
overall survival rates are relatively low (4), likely due to
acquired drug resistance. Therefore, it is necessary to
identify other agents or drugs that can be used against drug-
resistant CCA.
Ivermectin (IVM) is a derivative of avermectin and was
discovered in 1970. IVM is one of the most effective anti-
parasitic drugs and is widely used in human and veterinary
medicine (5). Apart from its anti-parasitic activity, IVM has
been shown to exert anti-cancer activities including
inhibition of proliferation as well as induction of apoptosis
and autophagy (6-8). The anticancer activities of IVM have
been observed both in vitro and in vivo in a number of solid
tumors such as colon cancer (9), glioblastoma (10), ovarian
cancer (11) and in hematological malignancies such as
leukemia (12). IVM has also been shown to reverse the drug
resistance of cancer cells (13-15). Therefore, IVM is a
promising anticancer agent and could possibly be used
against drug-resistant cancers.
It is not known whether IVM can exert anticancer activity
against CCA, and especially against drug-resistant CCA.
Herein, we explored the anti-CCA activity of IVM and
4837
Correspondence to: Prof. Somchai Pinlaor, Ph.D., Department of
Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen
40002, Thailand. Tel: +66 43348387, Fax: +66 43 202475, e-mail:
psomec@kku.ac.th
Key Words: Ivermectin, cholangiocarcinoma, drug resistance,
gemcitabine, KKU214
GemR
.
ANTICANCER RESEARCH 39: 4837-4843 (2019)
doi:10.21873/anticanres.13669
Anti-parasitic Drug Ivermectin Exhibits
Potent Anticancer Activity Against
Gemcitabine-resistant Cholangiocarcinoma In Vitro
KITTI INTUYOD
1,2
, CHARIYA HAHNVAJANAWONG
3
, PORNTIP PINLAOR
2,4
and SOMCHAI PINLAOR
1,2
1
Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand;
2
Cholangiocarcinoma Research Institute, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand;
3
Department of Microbiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand;
4
Centre for Research and Development in Medical Diagnostic Laboratory,
Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand

demonstrated that IVM inhibits CCA cell growth and
clonogenicity in dose- and time-dependent manner in both
gemcitabine-sensitive and gemcitabine-resistant CCA cell
lines in vitro. Interestingly, the anticancer properties of IVM
were more prominent in gemcitabine-resistant CCA cells.
These results suggest that IVM is a promising agent for
treatment of CCA.
Materials and Methods
Materials. Dulbecco’s Modified Eagle’s Medium (DMEM), sterile
phosphate-buffered saline (PBS), fetal bovine serum (FBS) and
penicillin-streptomycin were purchased from Gibco (Grand Island,
NY, USA). Trypsin-EDTA solution and flat-bottomed 96-well plates
were obtained from Corning (Corning, NY, USA). FxCycle
PI/RNase staining solution was obtained from Invitrogen (Thermo
Fisher Scientific Inc., Waltham, MA, USA). Ivermectin (IVM),
dimethyl sulfoxide (DMSO), trichloroacetic acid (TCA) and
sulforhodamine B (SRB) were purchased from Sigma Aldrich (St.
Louis, MO, USA). Gemcitabine (Gemzar) was purchased from Eli
Lilly (Indianapolis, IN, USA).
Human CCA cell lines. Gemcitabine-sensitive CCA KKU214 (16)
and gemcitabine-resistant KKU214 (KKU214
GemR
) cell lines (17)
were established from Thai CCA patients as described previously.
Both cell lines were maintained in DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at
37˚C with 10% CO
2
in a humidified incubator. KKU214
GemR
was
maintained in the presence of gemcitabine. Before use in an
experiment, this cell line was cultured in a drug-free medium for
one passage.
Assessment of cell proliferation. CCA cells seeded in flat-bottomed
96-well plates (2,000 cells/well) were treated with either DMSO
(control) or different concentrations of IVM dissolved in DMSO for
24, 48 and 72 h. Thereafter, the cells were fixed with cold 40% TCA
for 1 h in a refrigerator and washed 3 times with running tap water.
Then, the fixed cells were stained with 0.4% (w/v) SRB solution in
1% acetic acid for 1 h at room temperature. Excess SRB solution
was removed by washing with 1% acetic acid and SRB dye was
dissolved with 10 mM Tris buffer pH 10.5. Absorbance at 492 nM
was measured using an ELISA reader (Tecan group ltd., Männedorf,
Switzerland). Absorbance obtained from DMSO-treated cells was
used as control.
Clonogenic assay. Approximately 1,000 cells per well of CCA cell
lines were grown in 6-well plates and treated with different
concentrations of either gemcitabine or IVM. The culture medium
was changed every 2 days and cells were grown for approximately
2 weeks. The cells were then fixed with 4% paraformaldehyde,
stained with 0.5% crystal violet and dissolved with 33% acetic acid.
The absorbance at 620 nM was measured using an ELISA reader
(Tecan group ltd.).
Cell cycle analysis. One million CCA cells treated with either
DMSO (control) or 4 μM IVM for 48 h were washed with ice-cold
PBS and fixed with 70% ethanol overnight at –20˚C. After washing
twice with ice-cold PBS, 500 μl of FxCycle PI/RNase staining
solution were added and the cells were incubated at room
temperature in the dark for 30 min. Finally, stained cells were
detected using a BD FACSCanto II flow cytometer (BD biosciences,
San Jose, CA, USA) and data analyzed using BD FACSDiva
software (BD biosciences).
Statistical analysis. Data are expressed as mean±SD. Student’s t-test
was used to test differences between experimental groups. A value
of p<0.05 was considered statistically significant. Non-linear
regression analysis was carried out to calculate IC
50
. All statistical
analyses were performed using Graphpad Prism 7.0 for Mac
(GraphPad Software, Inc., CA, USA).
Results
IVM treatment suppressed proliferation of CCA cells and
displayed potent growth inhibitory activity against
gemcitabine-resistant CCA cells. Both gemcitabine-
sensitive (KKU214) and gemcitabine-resistant
(KKU214
GemR
) CCA cell lines (17) were treated with
different concentrations of IVM for up to 72 h. The SRB
assay showed that cell proliferation in both cell lines
ANTICANCER RESEARCH 39: 4837-4843 (2019)
4838
Figure 1. IVM treatment inhibited cell proliferation of KKU214 and KKU214
GemR
CCA cell lines. The anti-proliferative activity of IVM was
evaluated using the SRB assay. Both KKU214 and KKU214
G
emR
CCA cell lines were treated with different concentrations of IVM for (A) 24 h, (B)
48 h and (C) 72 h. DMSO-treated cells were used as controls. The experiment was performed in triplicate. IVM: Ivermectin.

decreased in dose- and time-dependent manner (Figure 1).
Potent cell proliferation inhibition (more than 75%) was
achieved in both CCA cell lines treated with 32 μM of IVM
for 48 h or 72 h (Figure 1B and C). Interestingly,
KKU214
GemR
cells, which are extremely resistant to
gemcitabine, were more sensitive to IVM treatment than
the parental KKU214 cell line. This was particularly
apparent at concentrations of IVM of 4 μM or higher, at all
time points. Non-linear regression analysis showed that the
IC
50
concentrations of IVM at 48 h and 72 h for KKU214
cells were 11.41 μM and 7.27 μM, respectively.
Corresponding values for KKU214
GemR
cells were 4.05 μM
and 3.15 μM, respectively (Figure 2). These findings
indicated that IVM exhibited anti-CCA potential, especially
for gemcitabine-resistant CCA cells.
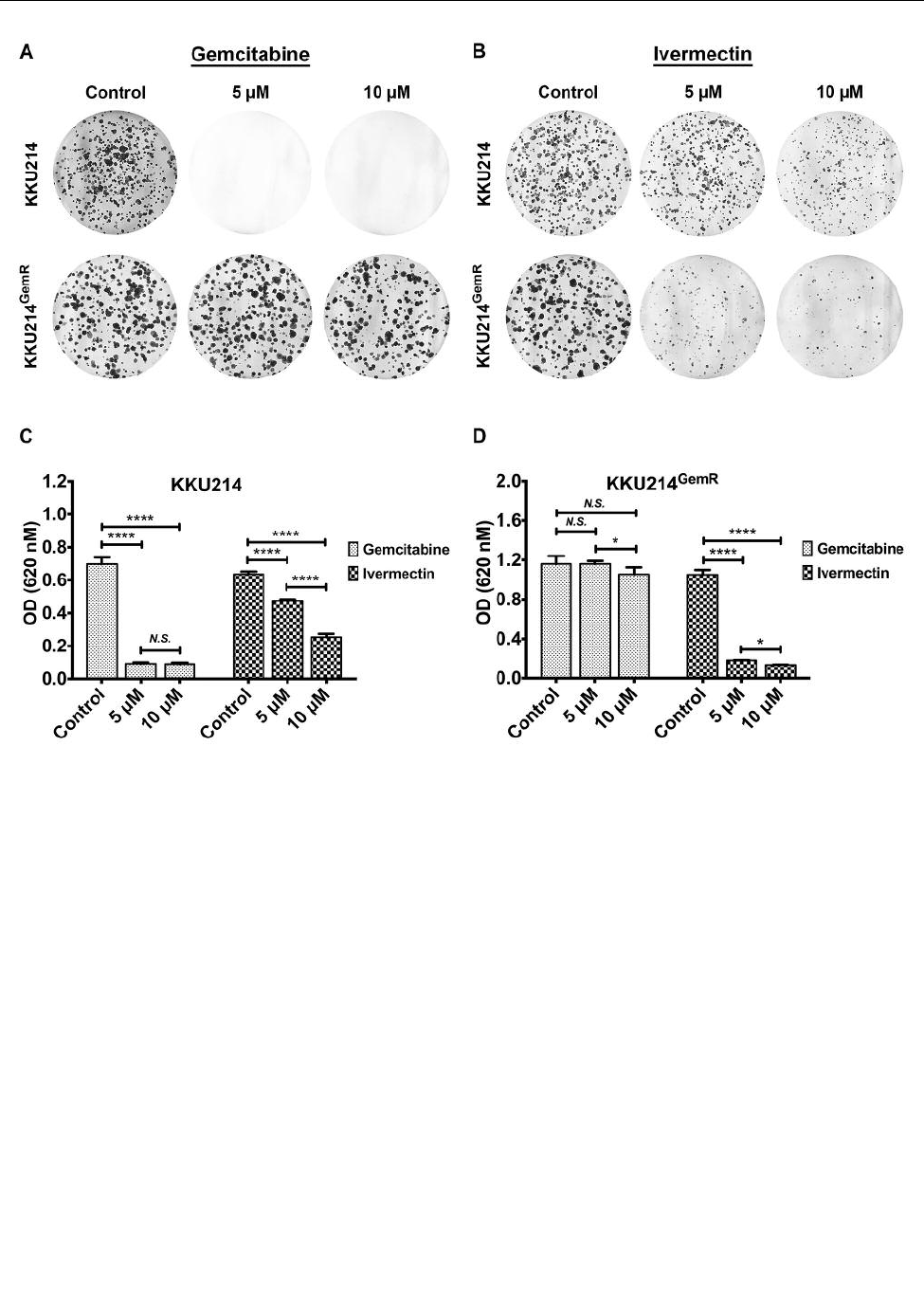
IVM treatment inhibited clonogenicity of CCA cells. A
clonogenic assay was employed to test the ability of IVM to
inhibit unlimited division and colony formation of CCA cell
lines (18). In line with the SRB assay, IVM treatment
significantly inhibited colony formation of KKU214 cells in
a dose-dependent manner compared to untreated controls
(p<0.0001), but was less effective than a similar dosage of
gemcitabine (Figure 3A-C). In contrast, gemcitabine
treatment was ineffective against the KKU214
GemR
CCA cell
line (Figure 3A and D), whereas IVM treatment significantly
suppressed colony formation of KKU214
GemR
cells
compared to untreated controls (p<0.0001, Figure 3B and
D). Once again, the inhibitory effect of IVM on colony
formation was more potent in KKU214
GemR
cells compared
to KKU214 cells. These results suggest that IVM exerts its
Intuyod et al: Anticancer Activity of Ivermectin Against Cholangiocarcinoma Cells
4839
Figure 2. Gemcitabine-resistant KKU214
GemR
cells were more sensitive to IVM treatment than gemcitabine-sensitive KKU214. Non-linear regression
analysis was performed to calculate the half-maximal inhibitory concentration 50 (IC
50
) of IVM at 48 h and 72 h for (A, B) KKU214 and (C, D)
KKU214
GemR
CCA cell lines. Data generated from the SRB assay were used for calculation.
anti-CCA activity partly through inhibition of unlimited
division and colony formation.
IVM treatment induced cell-cycle arrest of gemcitabine-
resistant KKU214
GemR
. Since the SRB assay demonstrated
the growth inhibition properties of IVM against CCA cell
lines, we then examined whether induction of cell-cycle
arrest contributed to this phenomenon. Flow cytometry
demonstrated that the cell-cycle profiles of the IVM-treated
KKU214 cells did not differ from those of controls (Figure
4A-C). However, treatment with 4 μM of IVM for 48 h
significantly induced cell-cycle arrest at the S phase in
KKU214
GemR
cells compared to controls (p<0.01, Figure
4D-F). Furthermore, the population of sub-G
0
/G
1
of IVM-
treated KKU214
GemR
cells, an indicator of apoptotic cell
death (19), was significantly higher compared to controls
(2.93±0.83 vs. 0.67±0.15, p<0.05, Figure 4D-F). Therefore,
in addition to inhibition of growth and colony formation,
IVM treatment can induce apoptotic cell death of
KKU214
GemR
cells.
Discussion
Resistance to conventional chemotherapy has been the most
important obstacle for effective treatment of many cancer
types including CCA. In addition to the increasing chance of
relapse, the development of cancer drug resistance could
possibly make treatment nearly impossible (20). A number
of therapeutic approaches to circumvent this problem have
been proposed such as targeted therapy (21), nanomedicine
ANTICANCER RESEARCH 39: 4837-4843 (2019)
4840
Figure 3. IVM treatment inhibited colony formation of KKU214 and KKU214
GemR
CCA cell lines. The effect of (A) gemcitabine treatment and (B)
IVM treatment on colony formation of both CCA cell lines was investigated using a clonogenic assay. Relative differences in colony formation of
(C) KKU214 and (D) KKU214
GemR
CCA cell lines treated with either gemcitabine or IVM compared to controls was investigated by measuring
absorbance at optical density (OD) 620 nM. The experiment was performed in triplicate. * and **** indicate a significant difference at p<0.05
and p<0.0001, respectively. N.S.: No significant difference.

(22), drug combination therapy using drugs with different
actions (23), etc. However, the efficacy of these approaches
for treatment of drug-resistant cancer has been questioned.
Herein, we demonstrated that the anti-parasitic drug IVM
exerts anticancer activity against CCA cells. Importantly,
IVM also exerted a potent anticancer activity against
gemcitabine-resistant CCA cells.
Due to its safety and high efficacy, IVM has been used for
treating both endo- and ectoparasites of animals and humans
for decades (24). IVM is also known to act against several
cancer types. A number of mechanisms have been found to
be associated with the anticancer activity of IVM. However,
an antiproliferative function has been widely reported (8). We
found that IVM treatment effectively inhibited cell
proliferation and colony formation of both gemcitabine-
sensitive and gemcitabine resistant CCA cell lines. Our
findings are in general agreement with a previous report
investigating colon cancer (9). Notably, non-linear regression
analysis found that the IC
50
of IVM against KKU214 cells
was higher than that in KKU214
GemR
cells. Increased
expression levels of chloride channels (12), which are usually
observed in drug resistant cancer cells (25-27), might be
responsible for the difference in susceptibility to IVM
between these cell lines. Furthermore, there might be a
difference in transcriptomic profile between gemcitabine-
sensitive and gemcitabine-resistant CCA cell lines (28). Cell-
cycle analysis found that IVM treatment induced cell death
of KKU214
GemR
cells as demonstrated by the significant
increase in the sub-G
0
/G
1
population. The induction of death
of KKU214
GemR
cells by IVM may be mediated by the
induction of mitochondrial dysfunction and oxidative DNA
damage as demonstrated in renal cancer cells (29) and
glioblastoma (10). However, significant cell-cycle arrest was
not observed in the KKU214 CCA cell line treated with 4 μM
of IVM for 48 h, consistent with the cell proliferation assay
in which treatment with 4 μM of IVM did not cause a
significant decrease in cell-proliferation rate compared to
controls. These results indicate that drug resistant cells are
more susceptible to IVM-induced cytostasis and cell death.
However, the precise mechanism by which IVM exerts
anticancer activity against CCA cells, especially drug resistant
cancer cells, remains to be elucidated.
Conclusion
IVM treatment exhibited anti-CCA potential as shown by
inhibition of cell growth and colony formation of CCA cell
lines. The anticancer activities of IVM were more potent in
gemcitabine-resistant KKU214
GemR
cells. Furthermore, IVM
treatment also induced S-phase and sub-G
0
/G
1
cell-cycle
Intuyod et al: Anticancer Activity of Ivermectin Against Cholangiocarcinoma Cells
4841
Figure 4. IVM treatment caused S-phase and sub-G
0
/G
1
cell-cycle arrest of KKU214
GemR
. Cell-cycle analysis of (A-C) KKU214 and (D-F)
KKU214
GemR
CCA cell lines after treatment with either DMSO (controls) or 4 μM of IVM for 48 h, was performed by flow cytometry. Cell-cycle
distribution was visualized using BD FACSDiva software. The experiment was performed in triplicate. * and ** indicate a significant difference at
p<0.05 and p<0.01, respectively. N.S.: No significant difference.

arrest in gemcitabine-resistant KKU214
G
emR
cells. Our
results highlight the therapeutic value of IVM: this drug
could be used for treatment of CCA, especially in patients
w
ho do not respond to gemcitabine treatment.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this
study.
Author’s Contributions
KI, CH, PP, and SP designed the experiments. KI performed the
experiments. KI, CH, PP, and SP performed the data analysis. KI
and SP drafted the manuscript. All Authors approved the final
version of manuscript for publication.
Acknowledgements
KI thanks the scholarship under the post-doctoral training program
from research affairs and graduate school, Khon Kaen University,
Thailand (Grant no. 60163) and also Khon Kaen University research
fund (Grant no. KKU61004405). The Authors thank Prof. David
Blair for critical reading and English editing of the manuscript.
References
1 Holohan C, Van Schaeybroeck S, Longley DB and Johnston PG:
Cancer drug resistance: An evolving paradigm. Nat Rev Cancer
13(10): 714-726, 2013. PMID: 24060863. DOI: 10.1038/nrc3599
2 Housman G, Byler S, Heerboth S, Lapinska K, Longacre M,
Snyder N and Sarkar S: Drug resistance in cancer: An overview.
Cancers (Basel) 6(3): 1769-1792, 2014. PMID: 25198391. DOI:
10.3390/cancers6031769
3 Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM,
Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz
MR and Wasan H: Guidelines for the diagnosis and treatment of
cholangiocarcinoma: Consensus document. Gut 51: VI1-9, 2002.
PMID: 12376491. DOI: 10.1136/gut.51.suppl_6.vi1
4 Thongprasert S: The role of chemotherapy in cholangio-
carcinoma. Ann Oncol 16: ii93-96, 2005. PMID: 15958484.
DOI: 10.1093/annonc/mdi712
5 Laing R, Gillan V and Devaney E: Ivermectin - old drug, new
tricks? Trends Parasitol 33(6): 463-472, 2017. PMID: 28285851.
DOI: 10.1016/j.pt.2017.02.004
6 Norenberg W, Sobottka H, Hempel C, Plotz T, Fischer W,
Schmalzing G and Schaefer M: Positive allosteric modulation
by ivermectin of human but not murine p2x7 receptors. Br J
Pharmacol 167(1): 48-66, 2012. PMID: 22506590. DOI:
10.1111/j.1476-5381.2012.01987.x
7 Polakis P: Wnt signaling and cancer. Genes Dev 14(15): 1837-
1851, 2000. PMID: 10921899.
8 Juarez M, Schcolnik-Cabrera A and Duenas-Gonzalez A: The
multitargeted drug ivermectin: From an antiparasitic agent to a
repositioned cancer drug. Am J Cancer Res 8(2): 317-331, 2018.
PMID: 29511601.
9 Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I and
Ruiz i Altaba A: The river blindness drug ivermectin and related
macrocyclic lactones inhibit wnt-tcf pathway responses in
human cancer. EMBO Mol Med 6(10): 1263-1278, 2014. PMID:
25143352. DOI: 10.15252/emmm.201404084
10 Liu Y, Fang S, Sun Q and Liu B: Anthelmintic drug ivermectin
inhibits angiogenesis, growth and survival of glioblastoma
through inducing mitochondrial dysfunction and oxidative stress.
Biochem Biophys Res Commun 480(3): 415-421, 2016. PMID:
27771251. DOI: 10.1016/j.bbrc.2016.10.064
11 Hashimoto H, Messerli SM, Sudo T and Maruta H:
I
vermectin inactivates the kinase pak1 and blocks the pak1-
dependent growth of human ovarian cancer and nf2 tumor
cell lines. Drug Discov Ther 3(6): 243-246, 2009. PMID:
22495656.
12 Sharmeen S, Skrtic M, Sukhai MA, Hurren R, Gronda M, Wang
X, Fonseca SB, Sun H, Wood TE, Ward R, Minden MD, Batey
RA, Datti A, Wrana J, Kelley SO and Schimmer AD: The
antiparasitic agent ivermectin induces chloride-dependent
membrane hyperpolarization and cell death in leukemia cells.
Blood 116(18): 3593-3603, 2010. PMID: 20644115. DOI:
10.1182/blood-2010-01-262675
13 Jiang L, Wang P, Sun YJ and Wu YJ: Ivermectin reverses the
drug resistance in cancer cells through egfr/erk/akt/nf-kappab
pathway. J Exp Clin Cancer Res 38(1): 265, 2019. PMID:
31215501. DOI: 10.1186/s13046-019-1251-7
14 Pouliot JF, L’Heureux F, Liu Z, Prichard RK and Georges E:
Reversal of p-glycoprotein-associated multidrug resistance by
ivermectin. Biochem Pharmacol 53(1): 17-25, 1997. PMID:
8960059. DOI: 10.1016/s0006-2952(96)00656-9
15 Furusawa S, Shibata H, Nishimura H, Nemoto S, Takayanagi M,
Takayanagi Y and Sasaki KI: Potentiation of doxorubicin-
induced apoptosis of resistant mouse leukaemia cells by
ivermectin. Pharm Pharmacol Comm 6(3): 129-134, 2000. DOI:
10.1211/146080800128735764
16 Tepsiri N, Chaturat L, Sripa B, Namwat W, Wongkham S,
Bhudhisawasdi V and Tassaneeyakul W: Drug sensitivity and
drug resistance profiles of human intrahepatic cholangio-
carcinoma cell lines. World J Gastroenterol 11(18): 2748-2753,
2005. PMID: 15884115.
17 Wattanawongdon W, Hahnvajanawong C, Namwat N,
Kanchanawat S, Boonmars T, Jearanaikoon P, Leelayuwat C,
Techasen A and Seubwai W: Establishment and characterization
of gemcitabine-resistant human cholangiocarcinoma cell lines
with multidrug resistance and enhanced invasiveness. Int J
Oncol 47(1): 398-410, 2015. PMID: 25998688. DOI: 10.3892/
ijo.2015.3019
18 Franken NA, Rodermond HM, Stap J, Haveman J and van Bree
C: Clonogenic assay of cells in vitro. Nat Protoc 1(5): 2315-
2319, 2006. PMID: 17406473. DOI: 10.1038/nprot.2006.339
19 Darzynkiewicz Z, Halicka HD and Zhao H: Analysis of cellular
DNA content by flow and laser scanning cytometry. Adv Exp
Med Biol 676: 137-147, 2010. PMID: 20687474. DOI: 10.1007/
978-1-4419-6199-0_9
20 Leary M, Heerboth S, Lapinska K and Sarkar S: Sensitization of
drug resistant cancer cells: A matter of combination therapy.
Cancers (Basel) 10(12), 2018. PMID: 30518036. DOI: 10.3390/
cancers10120483
21 Gao Y, Shen JK, Milane L, Hornicek FJ, Amiji MM and Duan
Z: Targeted cancer therapy; nanotechnology approaches for
overcoming drug resistance. Curr Med Chem 22(11): 1335-1347,
2015. PMID: 25666804.
ANTICANCER RESEARCH 39: 4837-4843 (2019)
4842

22 Markman JL, Rekechenetskiy A, Holler E and Ljubimova JY:
Nanomedicine therapeutic approaches to overcome cancer drug
resistance. Adv Drug Deliv Rev 65(13-14): 1866-1879, 2013.
PMID: 24120656. DOI: 10.1016/j.addr.2013.09.019
23 Al-Lazikani B, Banerji U and Workman P: Combinatorial drug
therapy for cancer in the post-genomic era. Nat Biotechnol
3
0(7): 679-692, 2012. PMID: 22781697. DOI: 10.1038/nbt.2284
24 Omura S: Ivermectin: 25 years and still going strong. Int J
Antimicrob Agents 31(2): 91-98, 2008. PMID: 18037274. DOI:
10.1016/j.ijantimicag.2007.08.023
25 Chen Q, Liu X, Luo Z, Wang S, Lin J, Xie Z, Li M, Li C, Cao
H
, Huang Q, Mao J and Xu B: Chloride channel-3 mediates
multidrug resistance of cancer by upregulating p-glycoprotein
expression. J Cell Physiol 234(5): 6611-6623, 2019. PMID:
30230544. DOI: 10.1002/jcp.27402
26 Shimizu T, Lee EL, Ise T and Okada Y: Volume-sensitive cl(–)
channel as a regulator of acquired cisplatin resistance.
Anticancer Res 28(1A): 75-83, 2008. PMID: 18383827.
27 Kischel P, Girault A, Rodat-Despoix L, Chamlali M,
Radoslavova S, Abou Daya H, Lefebvre T, Foulon A, Rybarczyk
P, Hague F, Dhennin-Duthille I, Gautier M and Ouadid-
Ahidouch H: Ion channels: New actors playing in chemothera-
peutic resistance. Cancers (Basel) 11(3), 2019. PMID: 30884858.
DOI: 10.3390/cancers11030376
28 Varamo C, Peraldo-Neia C, Ostano P, Basirico M, Raggi C,
Bernabei P, Venesio T, Berrino E, Aglietta M, Leone F and
Cavalloni G: Establishment and characterization of a new
intrahepatic cholangiocarcinoma cell line resistant to
gemcitabine. Cancers (Basel) 11(4), 2019. PMID: 30979003.
DOI: 10.3390/cancers11040519
2
9 Zhu M, Li Y and Zhou Z: Antibiotic ivermectin preferentially
targets renal cancer through inducing mitochondrial dysfunction
and oxidative damage. Biochem Biophys Res Commun 492(3):
373-378, 2017. PMID: 28847725. DOI: 10.1016/j.bbrc.2017.
08.097
Received August 23, 2019
Revised August 28, 2019
Accepted August 30, 2019
Intuyod et al: Anticancer Activity of Ivermectin Against Cholangiocarcinoma Cells
4843
