
Research Article
Leishmania survives by exporting miR-146a from infected
to resident cells to subjugate inflammation
Satarupa Ganguly
1,
* , Bartika Ghoshal
1,
*, Ishani Banerji
1,2,
* , Shreya Bhattacharjee
1,2
, Sreemoyee Chakraborty
1,2
,
Avijit Goswami
1
, Kamalika Mukherjee
1
, Suvendra N Bhattacharyya
1,2
Leishmania donovani, the causative agent of visceral leishman-
iasis, infects and resides within tissue m acropha ge cells. It is not
clear how the parasite infected cells crosstalk with t he non-
infected cells to regulate the infection process. During infection,
Leishmania adopts a dual strategy for its survival by regulating
the intercellular transport of host miRNAs to restrict inflam-
mation. The parasite, by preventing mitochondrial function of host
cells, restricts the entry of liver cell derived miR-122–containing
extracellular vesicles in infected macrophages to curtail the in-
flammatory response associated with miR-122 entry. On contrary,
the parasite up-regulates the export of miR-146a from the infected
macrophages. The miR-146a, associated with the extracellular
vesicles released by infected cells, restricts miR-122 production
in hepatocytes while polarizing neighbouring na
¨
ıve macro-
phages to the M2 state by affecting the cytokine expression. On
entering the recipient macrophages, miR-146a dominates the
miRNA antagonist RNA-binding protein HuR to inhibit the
expression of proinflammatory cytokine mRNAs having HuR-
interacting AU-rich elements whereas up-regulates anti-
inflammatory IL-10 by exporting the miR-21 to polarize the
recipient cells to M2 stage.
DOI 10.26508/lsa.202101229 | Received 8 September 2021 | Revised 7 February
2022 | Accepted 8 February 2022 | Published online 24 February 2022
Introduction
Leishmania donovani (Ld) is the causative agent of visceral leish-
maniasis that affects a large portion of the human population in the
Indian subcontinent and also in sub-Saharan Africa (Lenk et al, 2018).
The apicomplexan parasite has a dual-stage life cycle and lives in the
gut of the sandfly vector as promastigotes. The promastigote changes
to amastigote stage after entering the mammalian host macrophage
cells (Sunter & Gull, 2017). The promastigotes enter the mammalian
host with the saliva of the sandfly vector introduced during the blood
meal and subsequently make entry into the hepatic tissue where
they infect the Kupffer cells at the initial stage of infection before the
infection load is transferred to the spleen (Walker et al, 2014). The Ld
parasite lives in the tissue macrophages within a specialized sub-
cellular structure called parasitophorous vacuole and alters the
signalling components of the infected macrophages to polarize to
M2 stage. Ld ensures low expression of proinflammatory cytokines
like IL-1β and TNF-α, whereas the anti-inflammatory cytokines IL-10
and IL-4 expression get enhanced in the infected host (Mukherjee
et al, 2013). The status of the noninfected macrophages present in
the infection niche is not clear, but it is certain that the nonin-
fected macrophages present in the infection niche should not
get activated to ensure the overall dominance of an anti-
inflammatory res ponse that needs to b e maintained i n the in-
fected tissue. How the parasite, which remains within the specialized
vacuole structure o f the infected host macrophage, cross-
communicates with the resident noninfected macrophages to
suppress expression of inflammatory cytok ines is an important
question to explore. In this context, the extracellular signals derived
from resident noninfected macrophages and hepatocytes that can
activate the infected macrophage should also be counteracted
within the infected host cell milieu.
Extracellular vesicles (EVs) are released by different types of
mammalian cells that are used primarily by mammalian immune
cells to cross-communicate the cellular status across cell boundaries
(Regev-Rudzki et al, 2013; Fernandez-Messina et al, 2015). It is known
that the infected cell EVs can be used to transfer the parasite derived
and host factors to target specific pathways in neighbouring cells
to ensure establishment of systematic infection and its propaga-
tion (Regev-Rudzki et al, 2013; Kalluri & LeBleu, 2020). miRNAs are
important post-transcriptional regulators of gene expression that
are expressed in a cell ty pe and stage specificmannerinmam-
malian hosts (Bartel, 2018). miRNAs are also known to be com-
municated across the cell boundary to affect neigh bouring cell
fates. Therefor e, if communicat ed from the infected host cells,
miRNAs, a s an epigenetic signal, could alter the gene expression
process in neighbouring cells. The role of specific miRNAs in regu-
lation of expression of pro or anti-inflammatory pathway components
1
RNA Biology Research Laboratory, Molecular Genetics Division, Council of Scientific and Industrial Research (CSIR)-Indian Institute of Chemical Biology, Kolkata, India
2
Academy of Scientific and Innovative Research (AcSIR), CSIR-Human Resource Development Centre , (CSIR-HRDC) Campus, Ghaziabad, India
*Satarupa Ganguly, Bartika Ghoshal, and Ishani Banerji contrib uted equally to this work.
©2022Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 1of23
on 6 September, 2024life-science-alliance.org Downloaded from
http://doi.org/10.26508/lsa.202101229Published Online: 24 February, 2022 | Supp Info:

in mammalian immune cells has already been studied (Lindsay,
2008).
In this article, we have documented an extraordinary mechanism
that the internalized Ld adopts to ensure an anti-inflammatory
infection milieu in the infected liver of the mammalian host. The
internalized parasite prevents the proinfl ammatory response in the
infected macrophage by preventing the entry of hepatocyte derived
miR-122–containing EVs. The parasites achieve it through the de-
polarization of mitochondria of the host cells by enhancing the
expression of the uncoupler protein Ucp2 that restricts the entry of
the hepatic EVs into the infected cells and prevents miR-122 in-
duced inflammatory responses. We have also noted miR-146a up-
regulation in the infected cells and the excess miR-146a also gets
packaged into the EVs released by the infected macrophages. EVs
containing miR-146a enter the hepatocytes to restrict the pro-
duction of miR-122 there. The miR-146a–containing EVs are also
internalized by the noninfected macrophages that then get po-
larized to the M2 stage to express IL-10 in a miR-146a dependent
manner. HuR, the RNA binding protein with known miRNA antag-
onistic function, induces export of miR-21 out of macrophage cells
to cause high expression of miR-21 target IL-10 in the noninfected
cells in presence of miR-146a. Interestingly, HuR itself gets even-
tually repressed by high miR-146a. This in turn, reduces the HuR-
mediated stabilization and enhanced expression of ARE-containing
proinflammatory cytokines in the recipient neighbouring nonin-
fected macrophage cells. Thus, by cross communicating the in-
fected host cell–derived miR-146a, Leishmania ensures its own
survival by regulating both miR-122 and HuR in neighbouring he-
patocyte and na
¨
ıve macrophage cells, respectively.
Results
Secretion of miR-122 by activated hepatocytes
Lipopolysaccharide or LPS is an immunogen, derived from the cell
wall of the Gram-negative bacteria and is known to stimulate the
macrophage cells via activation of TLR4 receptor and p38/MAPK
pathway (Bode et al, 2012). LPS increases the expression of
proinflammatory cytokines by enhancing the NF-ĸβ–dependent
transcription and also by inac tivati ng the repressive miRNAs in
LPS-activated macrophages (Mazumder et al, 2013). In mammalian
liver, the LPS stimulation leads to activation of the tissue resident
macrophages, and thus, LPS increases liver inflammation (Rex
et al, 2019). Hepatocytes also respond to LPS and altered metabolic
function of the hepatocytes exposed to LPS has been documented
(Masaki et al, 2004; Momen-Heravi et al, 2015). miR-122 is the key
pro-inflammatory miRN A expressed in hepatocytes (Momen-
Heravi et al, 2015). We documented de creased miR-122 level in
mammalian liver cell H uh7 ex posed to LPS (Fig S1A and B). The
decreased miR-122 level was associated with enhanced phosp ho-
p38 MAPK level in LPS-treated Huh7 cells (Fig S1C). The decreased
cellular miR-122 was associated with increased miR-122 detected
in the EVs released by LPS-treated Huh7 cells (Fig S1D and E). What
consequence could this EV-associated miR-122 have on the tissue
resident macrophages infected with Leishmania?Uponinfection
of the host, the liver is the first tissue where the Ld initiates the
infection process by targeting the tissue macrophage the Kupffer
cells (Beattie e t al, 2010). Hepatic miR-122, secreted as part of EVs
from hepatocytes, can interact with macrophages to transfer the
miRNA to the resident macrophages and could enhance the ex-
pression of pro-inflammatory cytokines (Momen-Heravi et al,
2015). Therefore, the Ld-infected macrophages must adopt strategies
to combat this activation process to protect themselves from getting
killed by the im munostimulatory effect of hepatocyte secreted
miR-122.
Leishmania prevent internalization of proinflammatory miR-122
to the infected cells
Ld is known to affect miRNA machineries of host cells to ensure its
proliferation (Ghosh et al, 2013; Chakrabarty & Bhattacharyy a, 2017).
Interestingly, like what happens in LPS-activated cells, expression
of miR-122 also caused an increase in the TNF-α mRNA and protein
levels in RAW264.7 macrophage cells (Fig 1A and C). Expression of
iNOS and NO were also getting increased with miR-122 expression in
macrophages (Fig 1C). Conversely, when Ld infection of RAW264.7
cells expr essing miR-122 was followed, we found increased
proinflammatory cytokine TNF-α and low anti-inflammatory cyto-
kine IL-10 mRNA levels upon miR-122 expression there (Fig 1D and
E). The infection level of respective cells was also found to be
reduced when the macrophages received the miR-122 positive EVs
before the infection ( Fig 1F and G). Thus, the hepatic miR-122 has an
immuno-protective role, and when transferred via EVs released by
hepatic
cells could prevent infection of neighbouring liver mac-
rophage cells—the first target of invading Ld pathogen in the
mammalian host (Ghosh et al, 2013; Momen-Heravi et al, 2015;
Chakrabarty & Bhattacharyya, 2017 ). To survive, Ld must prevent
this EV-mediated miRNA transfer process to stop inflammatory
response in the host cells upon its interaction with miR-122 positive
EVs. We hypothesized that Ld could have hijacked the inflammatory
machinery of the host cell by preventing the miR-122–containing EV
transfer to infected macrophages, and thus could ensure survival
of the internalized pathogen. Consistent with the assumption, we
found an increased production of proinflammatory cytokines and
miR-155, the hallmark of inflammatory response in macrophage
cells upon treatment with miR-122 positive EVs. But in cells already
infected with Ld, the uptake of miR-122–containing EVs was found to
be significantly compromised with concomitant reduction in miR-
155 or TNF-α production in the infection context (Fig 1H). miR-
122–containing EV treatment, however, do not have any major effect
on cellular NO and iNOS level (Fig 1I).
Leishmania inhibits entry of miR-122–containing EVs in the
infected Kupffer cells in mouse liver
EVs packed with miR-122 were generated from mouse liver cells
HePa1-6. The miR-122–containing EVs were used to treat the mouse
primary macrophage to score the effect of Ld infection on in-
ternalization of miRNA in infected mouse primary macrophages
(Fig 2 A). We documented a substa ntial reduction in EV-mediated
miRNA entry in infected cells (Fig 2B). To score the same in vivo, we
used mice infected with Ld and measured the effect of infection
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 2of23

Figure 1. Hepatic miR-122 acts as an immunostimulant for mammalian macrophage and Ld restricts miR-122 entry in infected macrophage.
(A) Diagrammatic depiction of experiments done with Leishmania donovani (Ld)–infected or uninfected RAW264.7 cells treated with miR-122–containing extracellular
vesicles (EVs). The macrophage cells were either infected or noninfected with Ld for 6 h after which both the groups were either treated with miR-122–containing EVs or
left untreated. (B) Western blot analysis of RAW264.7 cells to show the infection related up-regulation of Ucp2 and down-regulation of HRS protein expression in RAW264.7
cells (left panel). Graphical representation of densitometric analysis of Ucp2 Western blot (right panel )(n ≥ 3 independent experiments, unpaired t test, P = 0.2139).
Values for uninfected and untreated cells were set as unit. (C) Effect of miR-122 overexpression on cytokine expression and nitric oxide generation in RAW264.7 cells.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 3of23

on EV-derived miR-122 entry in infected mouse liver macrophage
cells (Fig 2C). After infection of 30 d and 24 h of injection of miR-
122–containing EVs, the hepatocytes and Kupffer cells were
separated on a Percoll gradient and enrichment of each cell
population was monitored by following the expression of mRNAs
encoding the marker genes (Fig 2D). The levels of infection in both
control and EV-injected population were monitored by following
the kinetoplast DNA content (Fig 2E). In the u ninfected Kupffer
cells, there wa s internalization of miR-122 after treatment with
miR-122–containing EVs and a substantial increase in the ex-
pression of pro-inflammatory TNF-α mRNA in miR-122 EV-treated
cells was noted (Fig 2F and G). However, the Ld infection sub-
stantially reduces the entry of miR-122 EVs in infecte d mouse
Kupffer cells as both miR-122 and TNF-α expression was found to
be substantially low in infected state (Fig 2H and I).
Leishmania depolarizes mitochondria to prevent the entry of
miRNA-containing EVs
Ld is known to cause a robust change in endocytic pathway of the
host cells and alter components that are also found to be used for
endocytic entry or exit of miRNAs (Lievin-Le Moal & Loiseau, 2016;
Chakrabarty & Bhattacharyya, 2017). Proximity of endosomes with
Ld-containing parasitophorous vacuoles in infected macrophage
cells was noted (Fig S2A and B). The endosome maturation process
in Ld-infected cells is known to get affected (Scianimanico et al,
1999). We explored the status of Rab proteins in Ld-infected
macrophages and observed a decrease in endosomal protein
Hepatocyte growth factor-regulated tyrosine kinase substrate
(HRS) expression in infected cells (Fig 1B). However, there has
not been a major change in Rab5a or RILP expression with Ld in-
fection of RAW264.7 cells. Interestingly, treatment of cells with EVs
containing miR-122 has no significant effect on endosome number or
Rab5a expression in infected RAW264.7 cells. The infection also had
no effect on Dynamin 2 expression and thus cannot account for the
reduced miR-122 entry that is known to be a dynamin 2 dependent
process in mammalian cells (Fig S2B and C;[Ghoshal et al, 2021]).
It is known that Ld targets the mitochondrial dynamics and
activity by inducing depolarization of mitochondria and reducing the
mitochondria–ER–endosome interaction in infected cells (Chakrabarty
& Bhattacharyya, 2017). Mitochondrial uncoupler protein Ucp2 gets up-
regulated upon Ld infection (Fig 1B) suggesting a depolarized mito-
chondrial state in infected cells (Chakrabarty & Bhattacharyya, 2017).
Does mitochondrial depolarization affect EV-entry? Mitochondria can
interact with several cellular compartments, like ER and endosomes
(Klecke r e t al, 2014; Todkar et al, 2 019). The Ucp2 is a mitochondrial
uncoupling protein causing defects in oxidative phosphorylation
and
ATP s ynthesis by changing membrane potential across mi-
tochondrial membranes. Hence, it was anticipated that uncou-
pling of the mitochondrial potential can affect the internalization
of EV-derived miRNAs in mammalian cells as it is known to affect
endogenous miRNA activity (Chakrabarty & Bhattacharyya, 2017).
FH-Ucp2 was expressed in the recipient HeLa cells and FH-
Ucp2–expressing cells were incubated with miR-122–containing
EVs (isolated from HeLa cells expressing pmiR-122). Ucp2 over-
expression caused a disruption in the mitochondrial structures as
observed microscopic ally (Fig 3 A). The low level of internalization
and consequently a lower repression activity of t he transferred
EV-derived miRNAs were observed in FH-Ucp2–expressing re-
cipient HeLa cells (Fig 3B–D). It has been reported earlier that Ld
infection or the loss of mitochondrial membrane potential by FH-
Ucp2 are accompanied by reduced juxtaposition of ER and mi-
tochondria (Chakrabarty & Bhattacharyya, 2 017)(Fig S3A and B).
Interestingly, oligomycin treatment that affects the ATP con-
centration alone and FCCP treatment that disrupts membrane
potential of m itochondria without much effect on cellula r ATP
content, do not have any notable inhibitory effect on EV-mediated
miR-122 entry in recipient cells (Fig S3C). These data suggest in-
volvement of altered mitochondrial dynamics and mitochondrial
interaction of subcellular organelles in FH-Ucp2–expressing cells to
The RAW264.7 macrophage cells were either kept as control, transfected with miR-122 expression plasmid pmiR-122 or with miR-146a expression plasmid pmiR-146a or
treated with LPS (as positive control) to determine the mRNA level of TNF-α (C, left panel, n = 3 independent experiments; P = 0.0361, 0.0084) and iNOS (C, middle right panel,
n = 3 independent experiments; P = 0.0562, 0.0002) from cellular RNA. Protein level of TNF-α (C, middle left panel, n = 3 independent experiments; unpaired t test, P =
0.0006) and nitric oxide level (right panel, n = 3 independent experiments; unpaired t test, P ≤ 0.0001, 0.0405, <0.0001) was also measured from culture supernatant.
Quantification of TNF-α mRNA levels by qRT-PCR was done for the conditions described above. 18s RNA or GAPDH mRNA was used as endogenous control for qRT-PCR.
Values for control plasmid transfected untreated cells were considered as unit for qRT-PCR (control). (D) Effect of miR-122 expression on Ld infection. This panel shows
the schematic model for RAW264.7 cells transfected with miR-122 expression plasmid (pmiR-122) followed by L. donovani infection. (E) Comparison of the
proinflammatory TNF-α, and anti-inflammatory IL-10 cytokine expression in pmiR-122 transfected or pCIneo control vector transfected RAW264.7 cells followed by infection
with Ld. An increase in the proinflammatory cytokine TNF-α ( P = 0.0180) and a decrease in the anti-inflammatory cytokine IL-10 (P = 0.0422) were observed in the presence
of miR-122 (n = 3 independent experiments).18s rRNA was used as endogenous control. Values for pCIneo control transfected and infected RAW264.7 cells were
considered as unit. (F) Microscopic analysis of Ld infection in RAW264.7 cells treated or untreated with miR-122–containing EVs. Cells were then visualized under the
confocal microscope. The Leishmania protein GP63 imaged with indirect fluorescence (red) and cells with internalized parasites were counted. Scale bar 8 μm. Marked
areas are zoomed for 5×. (G) Effect of miR-122–containing EVs on Ld infection. Graphical representation of the percent of RAW264.7 cells infected with L. donovani as
observed microscopically (n = 132, number of cells; P = 0.0038, unpaired t test). (H) Quantification of miRNA levels upon Ld infection followed by EV treatment in RAW264.7
cells. Real-time PCR showed no increase in miR-122 levels in infected macrophages incubated with miR-122 positive EVs (top left panel
; P =
0.0215, 0.3211, 0.3808). miR-155
levels were also quantified under similar conditions (bottom left panel; P = 0.0024, 0.0736, 0.1368) (n = 4 independent experiments). U6 was used as endogenous control.
Relative levels of cytokine mRNA were also quantified. The proinflammatory cytokine, TNF-α (top right panel; P = 0.0142, <0.0001, 0.0013) and IL-1β (bottom right panel; P =
0.0438, <0.0001, 0.0366) did not increase in presence of the parasite followed by EV treatment (n = 4 independent experiments). 18s rRNA was used as endogenous control.
Values for uninfected cells were set as unit. (I) Quantification of iNOS mRNA level and Nitric oxide (NO) generation after miR-122–containing EV treatment of RAW264.7
cells. Nitric oxide level was determined from culture supernatant of EV-treated RAW264.7 cells (upper panel, n = 3 independent experiments, unpaired t test; P = 0.3878).
qRT-PCR analysis was used to determine the relative level of iNOS mRNA in recipient RAW264.7 cells (lower panel, n = 3 independent experiments; P = 0.1275). GAPDH mRNA
was used as endogenous control. Values for control EV-treated cells were considered as unit for qRT-PCR. Data information: In all experimental data, error bars are
represented as mean ± SEM, ns, nonsignificant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, respectively. P-values were calculated by two-tailed paired t test in most of
the experiments unless mentioned otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 4of23

Figure 2. miRNA uptake is prevented in Ld -infected primary macrophages and Kupffer cells.
(A) A model to show the effect of Leishmania infection on uptake of miR-122–containing extracellular vesicles (EVs) in primary peritoneal macrophages. Macrophages
were either infected or noninfected with Ld followed by miR-122–containing EV treatment. The EVs were obtained from miR-122 expressing murine hepatic cell HePa 1–6.
(B) Relative levels of internalized miR-122 in infected or noninfected primary macrophages (n = 3 independent experiments; 0.0379, 0.0033, 0.2726, Unpaired t test P =
0.0227). U6 was used as endogenous control and values for uninfected control cells were considered as unit. (C) Schematic representation of Ld infection of mice
followed by miR-122–containing EV treatment. Mice were infected with Ld for 30 d followed by tail vein injection of miR-122–containing EVs. After 24 h of treatment, mice
were euthanized and Kupffer cells were isolated to quantify the miR-122 content. (D) Characterisation of isolated hepatocytes and Kupffer cells. Quantification of
hepatocyte specific albumin mRNA in hepatocytes and Kupffer cells to show their low levels in Kupffer cell isolate (left panel; P = 0.143, n = 4 number of mice). Relative
levels of macrophage specific C-type Lectin Domain Family 4, Member F (Clec4f) mRNA between hepatocytes and Kupffer cells showing high levels in Kupffer cells (right
panel; P = 0.0378, n = 4 number of mice). GAPDH was used as endogenous control. Values for hepatocytes were considered as unit. (E) Comparison of normalized Ct values of
Kinetoplast DNA in infected or uninfected Kupffer cells isolated from animals untreated or treated with EVs containing miR-122 to determine the parasitic load in
Kupffer cells (n = 3 number of mice; P = 0.0009, 0.0005, unpaired t test). GAPDH was used as endogenous control. (F ) Mean Ct values of internalized miR-122 in
uninfected Kupffer cells either untreated or treated with miR-122–containing EVs (n = 3 number of mice; P = 0.00 49, unpaired t test). (G) Mea n Ct values of TNF-α
mRNA in uninfected Kupffer cells upon treatment with miR-122–EVs compared with untreated Kupffer cells (n ≥ 2; P = 0.0444, unpaired t test). (H) The internalization of
EV-derived miR-122 declined in the presence of Leishmania infection. Real-time PCR revealed the relative levels of miR-122 in uninfected or infected Kupffer cells isolated
from animals treated with miR-122–containing EVs (n = 3 number of mice; P = 0.0162). U6 was used as endogenous control and values for uninfected miR-122 EV-treated
cells were considered as unit. (I) Relative levels of proinflammatory cytokine TNF-α
in Leishmania infecte
d or uninfected miR-122–EV-treated animals’ Kupffer cells
(n = 3 number of mice; P < 0.0001). GAPDH mRNA was used as endogenous control and values for uninfected miR-122 EV-treated set were considered as unit. Data
information: In all experimental data, error bars are represented as mean ± SEM, ns, nonsignificant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.000 1, respectively. P-values
were calculated by two-tailed paired t test in most of the experiments unless mentioned otherwise.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 5of23

Figure 3. Mitochondrial depolarization and ER detethering by Ld prevents internalization and activity of extracellular vesicle (EV)-derived miRNAs in recipi ent cells.
(A) Structure of mitochondria (green) in Mito-GFP–expressing HeLa cells transfected with FH -Ucp2 (red) or control plasmid. Scale bar 5 μm. (B) Graphical representation
of the internalization of CD63-GFP–positive EVs in recipient HeLa cells transfected either with control or FH-Ucp2–expression plasmids (n = 18, number of cells; P < 0.0001,
unpaired t test). (C) Effect of FH-Ucp2 expression on uptake of miR-122. Relative level of internalized EV-derived miR-122 in recipient HeLa cells transfected either with
control or FH-Ucp2 expression plasmids (left panel; n = 3 independent experiments; P < 0.0001). U6 was used as endogenous control and values for control plasmid
transfected and miR-122 EV-treated cells was considered as unit. Right panel shows the Western blot for HA. β-Actin was used as loading control. (D) Luciferase assay to
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 6of23

cause the defect in cellular uptake of miRNA-containing EVs in
mammalian cells. Mfn2 is a protein responsible for mitochondrial
tethering with ER. Mfn2 knockout MEFs showed a decreased ER
tethering of mitochondria. To revalidate that the Ucp2 over-
expression mediated loss of ER–mitochondria contact is respon-
sible for the reduced EV-associated miRNA internalization and Ago2
association, EV-derived miR-122 levels were compared in Mfn2
knockout and wild-type MEFs. Importantly, as reported earlier, the
endogenous miR-16 level was found to be increased in Mfn2 de-
pleted cells (Fig 3E and F) that has been consistent with the pre-
vious findings on increased cellular miRNA content upon Ucp2
overexpression (Chakrabarty & Bhattacharyya, 2017). To reconfirm
the importance of Ucp2-lowering for cellular entry of EV-associated
miRNAs we used genipin, an inhibitor of Ucp2, to see its effect on
EV-mediated miR-122 entry. The miR-122 entry and associated
increase in T NF-α expressi on has been observed in infected
macrophage cells treated with Ucp2 inhibitor genipin ( Fig 3G).
Interestingly, ectopic expression of FH-Ucp2 has a marginal effect
on low endogenous levels of miR-122 expressed in RAW264.7 cells
compared to the changes in miR-122 levels in pmiR-122 transfected
RAW264.7 cells (Fig S3D). Conversely, there has been no detectable
change in the cellular ATP level or mitochondrial polarization status
or Ucp2 expression in RAW264.7 cells ectopically expressing miR-122
from pmiR-122 plasmid (Fig S3E–G). Overall, these data suggest Ld
infection–associated increase in Ucp2 that causes mitochondrial
detethering with ER and prevents the pro-inflammatory miR-122
entry via EVs into the infected macrophage to stop expression of
pro-inflammatory cytokines (Fig 3H).
Leishmania infection increases cellular and extracellular
miR-146a levels in infected cells
The hypothesis that the infected cells should release the EVs
enriched with factors to facilitate the propagation of infection
inspired us to examine the key protein components that are
specifically released by infected cells or their export is prevented in
the infection context. The EVs isolated from control and Ld-infected
cells were analysed for their protein content. Although upon in-
fection there has been a drop in expression of HRS or Rab27a
proteins that are known to have role in late endosome/MVB for-
mation and EV release (Fig 4A and B), we did not detect a significant
change in EV diameter or marker protein levels in population
isolated from both control and Ld-infected RAW264.7 cells (Fig
4C–E). In the mass spectrometric analysis of released EVs that is
followed by candidate protein identification, we have documented
several candidate proteins that showed exclusive presence in the
EVs isolated from control or Ld-infected cells. However, differential
expression of any important regulatory factors of cytokine ex-
pression, were not detected (Fig S4 and Tables S1–S3). Therefore, it
could be the small RNA population of the infected host that may
carry the required anti-inflammatory information to the neigh-
bouring cells as part of EVs. In infected cells, both miR-146a and
miR-155, two most important regulatory miRNAs of inflammation
pathways, were found to be up-regulated. Interestingly, among
them, only miR-146a were detected in EVs released by infected cells
and the content of miR-146a had increased several folds in EVs
from Ld-infected cells compared with EVs from control noninfected
cells (Fig 4F and G). Similar increase in miR-146a in EVs released by
infected primary macrophage was also detected (Fig 4H). The
miRNAs that are reported to be increased in Ld-infected cells
(Chakrabarty & Bhattacharyya, 2017), miR-21, miR-125b, or miR-16 all
were fou nd to be incr eased in the E Vs rel eased by infect ed ce lls
(Fi
g 4I).
Leishmania by targeting HuR ensures export of miR-146a but not
miR-155 from the infected cells
We report differential export of miR-146a from Ld- infected
RAW264.7 macrophage. But how miR-146a gets differe ntially
exported out of infected macrophage cells is a fascinating question.
Retention of miRNA and their targets with polysomes has been
found to be the reason for reduced miRNA export noted in
mammalian cells (Ghosh et al, 2015), whereas target RNA presence
positively influences the export process (Ghosh et al, 2021). We have
isolated the polysomes from control and infected cells and de-
tected increased retention of both miR-146a and miR-155 with
polysomes in the infected cells (Fig S5A and D). The increased
retention of IL-10 and MyD88 mRNAs, known to get expressed in
infected cells, were also detected more with polysomes isolated
from the infected cells but the miR-146a target TRAF6 mRNA and
TNF-α mRNA was found to be less with infected cell polysomes (Fig
S5B and C).
show the repression levels of RL-perf-miR-122 in recipient cells transfected with pCIneo control or FH-Ucp2–expression plasmids and treated with miR-122–containing
EVs. The repression levels were calculated as a ratio of firefly normalized RL-Control value to firefly normalized RL-perf-miR-122 value (n = 3 independent experiments;
0.0117, 0.0987, unpaired t test = 0.0013). (E) Relative level of internalized miR-122 (left panel; P = 0.0012) and endogenous miR-16 levels (right panel; P = 0.0316) in Mfn2
(Mitofusin2) wi ld-type and knockout MEF cells treated with miR-122–containing EVs. Quantification was done by qRT-PCR and U6 levels were used as normalizing control
(n = 3 independent experiments). Values for Mfn2 wild-type cells were set as unit. (F) FH-Ago2 associated miR-122 levels in Mfn2 wild-type and knockout MEF cells
(transfected with FH-Ago2) co-cu ltured with miR-122 expressing HeLa cells. Immunoprecipitated Ago2 levels were used for normalization of associated miR-122 (n = 3
independent experiments; P = 0.0077). Values for Mfn2 wild-type cells was considered as unit. (G) Rescue of EV-miR-122 internalization in Leishmania donovani infected
RAW264.7 cells in presence of genipin, the inhibitor of Ucp2. RAW264.7 cells infected with Ld were treated with genipin (100 μM after 4 h of infection) followed by addition of
miR-122 positive EVs. Relative levels of miR-122 (top panel; P = 0.0311) and TNF-α mRNA (bottom panel; P = 0.0968) increased in infected RAW264.7 cells treated with genipin
(n = 3 independent experiments). U6 levels were used as control for normalization of miRNA and 18s rRNA was used normalizing levels for mRNA. Values for infected and
genipin untreated set was consider ed as unit. (H) Diagrammatic representation of the EV-mediated crosstalk between hepatocytes and macrophages. Hepatocytes release
miR-122–containing EVs which can be transferred to macrophag es that causes production of proinflammatory cytokines and can prevent Ld infection (red arrow).
Inversely, Ld-infected macrophages are unable to take up the miR-122–containing EVs due to up-regulation of Ucp2 protein in cells and are tuned to have high
production of anti-inflammatory cytokines for sustained parasitic infection (green arrow). Data information: In all experimental data, error bars are represented as mean ±
SEM, ns, nonsignificant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, respectively. P-values were calculated by two-tailed paired t test in most of the experiments unless
mentioned otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al.
https://doi.org/10.26508/lsa.202101229 vol
5 | no 6 | e202101229 7of23

Figure 4. Leishmania donovani (Ld) infection triggers extracellular export of miRNAs from infected macrophage cells.
(A) Schematic representation of the experiment. After 24 h of Ld infection, the host macrophage and extracellular vesicles (EVs) isolated from culture supernatant were
analysed for cellular and EV-associated protein and RNA analysis. (B) Ld infection altered cellular protein levels. Western blot images showed protein le vels after 24 h of
infection of macrophages. β-Actin was used as loading control. (C, D, E) Characterisation and quantification of EVs released from control and Ld-infected macrophages.
Nanoparticle Tracking Analysis (NTA) of EVs released from control (C, left panel) and infected cell (C, right panel). (D) Western blot images showed EV marker proteins in
control and infected cell released EVs. (E) Number and size of EVs released by control and infected cells were quantified by NTA analysis (E, left panel, P = 0.0008 and right
panel, P = 0.0677, n = 3 independent experiments, respectively, unpaired t test). (F, G) miR-146a level increases and the miRNA gets exported from Ld-infected macrophage
cells. qRT-PCR based relative quantification of cellular miR-155 (F, left panel, P = 0.0817, n = 3 independent experiments) and miR-146a (F, right panel, P = 0.0146, n = 4
independent experiments) after 24-h infection of RAW264.7 cells. U6 was used as endogenous control. miRNA levels of infected cells were normalized against noninfected
controls. qRT-PCR data represented the relative level of miR-146a and miR-155 in EVs from RAW264.7 cells after infection. EV marker protein Alix was used for normalization
of EV-associated miR-146a level (G, P = 0.0229, n = 4 independent experiments). Values for uninfected cell–derived EVs were set as unit. (H) Ld infection triggers the
extracellular export of miRNAs from mouse peritoneal macrophages. miR-146a level was also estimated in EV-derived from mouse peritoneal macrophage(H,upper
panel, P = 0.0006, n = 5 independent experiments, unpaired t test) and Western blot data also showed EV marker proteins (H, lower panel). (I) Average Ct value of miR-21 (I,
left panel, P = 0.0011, n = 3 independent experiments, unpaired t test), miR-125b (I, middle panel, P = 0.2654, n = 3 independent experiments, unpaired t test), and miR-16 (I,
right panel, P = 0.0794, n = 3 independent experiments, unpaired t test) in EVs released from Ld-infected and noninfected RAW264.7 cells. Data information: In all
experimental data, error bars are represented as mean ± SEM, ns, nonsignificant,
*P < 0.05, **P < 0.01, ***P < 0.001, respectively. P-values were calculated by two-tailed
paired t test in most of the experiments unless mentioned otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 8of23

The polysome retention data thus could not clearly explain why
there has been a preference of miR-146a for export from Ld-
infected cells. miRNAs accumulate at endosomes before they get
packed and exported out (Mukherjee et al, 2016). We had isolated
the endosomes and ER fractions from control and infected cells to
document preferential enrichment of miR-146a over miR-155 at the
endosomal fraction that can explain its enhanced export observed
in infected macrophage cells (Fig S5E–G). HuR is known for its role
in export of miRNAs in mammalian liver and macrophage cells
(Mukherjee et al, 2016). HuR drives miRNA accumulation with
endosomes before the export but HuR level is known to get reduced
in Ld-infected cells (Goswami et al, 2020)(Fig S5H). The HuR level
increases after LPS treatment that is associated with enhanced
export of miR-155 from LPS-activated macrophage cells (Goswami
et al, 2020), it is possible that low HuR in Ld-infected cells may cause
reduced export of miR-155. Does binding of HuR makes miR-155
available in endosomal fraction and for export? Supporting the
notion, we have documented strong interaction of HuR with miR-
155 in LPS-treated cells (Fig S5I and J). Similarly export of miR-155
from HA-HuR expressing cells has also been noted (Fig S5K ).
EV-containing miR-146a down-regulates miR-122 maturation in
hepatocytes
The infected cells prevent the entry of miR-122–containing EVs
thereby restricting the activation of the Ld-infected cells by miR-122
to prevent the death of the internalized parasites owing to en-
hanced proin flammatory response observed in miR-122 recipient
macrophages (Chen et al, 2011). However, miR-122 in hepatic EVs
could also get transferred to a na
¨
ıve neighbouring macrophage not
infected with Ld. How the parasite prevents the activation of resident
neighbouring na
¨
ıve macrophages to prevent the proinflammatory
cytokine accumulation in the infection microenvironment? It is likely
that Ld must have adopted a mechanism to reduce the miR-122–
containing EV release by neighbouring hepatocytes in the infection
niche to prevent miR-122–mediated activation of na
¨
ıve noninfected
macrophage cells. Can Ld doit through cross talk with hepatocytes
via the EVs released by infected cells? We have noted miR-146a
as the predomina nt anti-inflammatory miRNA in the EVs se-
creted by the infected macrophage (Fu et al, 2017). Does miR-
146a reduce the miR-122 level in the hepatocytes?
We have isolated the EVs from infected macrophage for treating
human hepatoma cell Huh7. We have documented an increase in
miR-146a content of recipient hepatic cells with a reduction in
mature miR-122 level there (Fig 5A and B). The decrease of mature
miR-122 was accompanied by an increase in pre-miR-122 level and
with an increase in endogenous control miR-16 level in the he-
patocyte. Thus, the miR-146a–mediated lowering of hepatic miR-122
is a miRNA specific effect. To confirm that the effect of miR-146a on
mature miR-122 level is miR-146a specific, we have isolated the EV
from RAW264.7 cells ectopically expressing miR-146a and used the
isolated EVs for the treatment of the hepatocytes (Fig 5C). The EVs
isolated from control and miR-146a expression plasmid transfected
cells were analysed by NTA to document no major change in size
and number of the EVs under miR-146a overexpression condition
(Fig S6A). Increased miR-146a content in EVs released from pmiR-
146a–expressing cells was also noted (Fig S6B) and cellular and EV
fractions were Western blotted for EVs and cytosolic markers to
confirm the purity of the fractions (Fig S6C). The miR-146a–
containing EV treatment enhanced cellular miR-146a level in re-
cipient hepatocytes with concomitant decrease in mature miR-122
and increase in pre-miR-122 levels observed there (Fig 5D). To
substantiate the idea of miR-146a–induced reduction in miR-122
levels in hepatocytes, we have expressed miR-146a in hepatocytes
and documented decreased miR-122 level associated with in-
creased pre–miR-122 and miR-146a levels in Huh7 cells (Fig 5E).
WehavenotedareductioninDicer1leveluponLd-infected cell
EV treatment of hepatocyt es. However, mi R-146a–c ontai ning EV
treatment or miR-146a expression in hepatocytes, did not alter
Dicer1 level (Fig 5F–H). Thus, altered pre–miR-122 processing by
Dicer1 in miR-146a enriched hepatocyte could not be explained
by the unchanged Dicer1 level. The increased pre–miR-122 however
got associated with the Dicer1 to a lesser extent in Huh7 cells
expressing pmiR-146a where total pre–miR-122 level increases (Fig
5J and K). Therefore, there must be a mechanism that excludes the
pre–miR-122 association with Dicer1 for its subsequent processing
to the mature form. The miR-146a expression, however, enhances
the P-ERK1/2 level and a decrease in P-p38 level (Fig 5I), suggesting
a possible lowering of transcriptional events for pre-miR-122 known
to be linked with P-p38 level (Basu & Bhattacharyya, 2014).
Infected cell secreted EVs downgrade inflammatory response in
neighbouring macrophage and induce macrophage polarization
Although lowering of miR-122 levels in hepatocytes could be one of
the mechanisms to reduce the proinflammatory response in the
na
¨
ıve macrophage, there must be additional mechanisms to restrict
the production of inflammatory cytokines in the neighbouring na
¨
ıve
macrophage cells adjacent to the infected macrophages. To score
the effect of infected cell EVs on na
¨
ıve macrophage cells, we treated
na
¨
ıve RAW264.7 cells with EVs isolated from Ld-infected RAW264.7
cells (Fig 6A). Interestingly, when the na
¨
ıve RAW264.7 cells were
pretreated with Ld
-infected cell–derived
EVs, we documented a
lesser responsiveness and relatively low proinflammatory cytokine
production in the treated cells when exposed to pro-inflammatory
agents such as LPS. Remarkably, the microvesicles (MVs) isolated
from the infected cells increase the production of cytokines in
recipient cells on LPS exposure (Fig 6B).
To score the effect of infected cell EVs on polarization of na-
ive macrophage, we treated the RAW264.7 macrophage with Ld-
infected EVs (Fig 6C) and measured the amount of internalized miRNA
and found an increase in miR-146a content. This was accompanied
by a reduction in iNOS expression (Fig 6D). With EV treatment, we
noted no change in Dicer1 level and a decrease in HuR expression
with increased levels of P-p38 and P-ERK1/2 levels (Fig 6E). These
were accompanied by an increase in IL-10 expression and decrease
in expression of proinflammatory cytokine IL-1β (Fig 6F). To con-
clude on casualty of EV-associated miR-146a with changed cytokine
expression observed in Ld-infected cell–derived EV-treated cells,
we used EVs released by pmiR-146a–expressing RAW264.7 cells and
treated na
¨
ıve RAW264.7 cells with miR-146a–containing EVs. We
noted an increase in IL-10 and a decrease in IL-1β as well as iNOS
mRNAs after miR-146a–containing EV treatment (Fig 6G and H).
Increased expression of miR-146a has also found to be associated
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 9of23

Figure 5. Extracellular vesicle (EV)-mediated transfer of Ld-infected cell derived miR-146a reduces miR-122 level in hepatic cells.
(A, B) Effect of control and Ld-infected cell–derived EVs on na
¨
ıve hepatocytes. (A) A schematic representation of the experiment (A). (B) Relative levels of miR-146a (B, left panel,
P = 0.0329, n = 3 independent experiments), miR-122 (B, middle left panel, P = 0.0364, n = 4 independent experiments), Pre-miR-122 (B, middle right panel, P =0.0993,n=3
independent experiments), miR-16 (B, right panel,unpairedt test P = 0.2074, n = 3 independent experiments) were measured by qRT-PCR in recipient hepatic Huh7 cells after 24 h of
control and infected cell–derived EV treatment. U6 was used as endogenous control for miRNA normalization and for Pre-miR-122 level, GAPDH was used as endogenous control.
Values for control EV-treated cells were used for normalization of infected cell–EV-treated cells. (C, D) Effect of pmiR-146 a–expressing macrophage derived-EV treatment on
recipient hepatocytes. (C) A schematic representation of the experiment has been described in panel. (D) Relative levels of miR-146a (D, left panel, P = 0.0213, n = 3 independent
experiments), miR-122 (D, middle panel, P = 0.0036, n = 3 independent experiments) and Pre-miR-122 (D, right panel, P = 0.0307, n = 3 independent experiments) were measured by
qRT-PCR in recipient Huh7 cells after pCIneo and pmiR-146a transfected RAW264.7 cell–derived EV treatment for 24 h. U6 was used as endogenous control for cellular miRNA level
normalization. Values for pCIneo-transfected cell–derived EV-treated cell were set as unit. For Pre-miR-122 level, GAPDH was used as endogenous control. (E) Effect of pmiR-146a
overexpression on miR-122 level in hepatocytes. Relative levels of miR-146a (E, left panel, P = 0.2251, n = 3 independent experiments), miR-122 (E, middle panel, P = 0.0003, n = 4
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 10 of 23

with decreased iNOS mRNA and NO levels in RAW264.7 cells (Fig 1C)
associated with a decrease in TNF-α protein level (Fig 1C) and cellular
IL-1β protein expression (Fig S6D), respectively. We expressed miR-
146a in na
¨
ıve and Ld-infected RAW264.7 cells and have noted a
decrease in IL-1β and TNF-α expression and increase in IL-10 ex-
pression (Fig 6I). Interestingly, inhibition of miR-146a by anti-miR-
146a reduced the expression of IL-10 both in uninfected and
Ld-infected cell–derived EV-treated RAW264.7 cells, whereas the ac-
tivation of miR-146a–expressing cells with LPS have a induced effect
on anti-inflammatory cytokine IL-10 mRNA level (Fig 6J and K). This
data suggests an M2 polarization of macrophage expressing miR-
146a and that become refractory to immuno-stimulation by LPS (Fig
6I–K). To further reaffirm the contribution of Ld-derived factors
associated with EVs-released by infected cells on na
¨
ıve macro-
phage cell polarization and IL-10 production, we treated RAW264.7
cells with Ld-derived EVs and lipophosphogylcan (LPG) isolated
from the Ld membrane. We have noted no important change in the
expression of major signalling component in Ld-derived EV-treated
cells (Fig S7A, B, and F). As reported earlier, we had noted decreased
TNF-α expression in both sets of treated cells, whereas IL-1β only
got reduced in presence of Ld-derived EVs but not with Ld LPG (Fig S7C
and E,[de Carvalho et al, 2019]). The IL-10 level decreased in both Ld-
derived EVs or LPG treatment and therefore, these Leishmania-derived
factors cannot be the major player for the observed increase in IL-10 in
neighbouring macrophage cells (Fi g S7 C and G). iNOS mRNA level,
however, decreases with Ld-derived EV treatment (Fig S7B), whereas
the Ld-derived EV, LPG, or soluble antigens of Leishmania (SLA) has an
insignificant effect on endogenous level of miR-122 in RAW264.7 cells
(FigS7D,E,andG). On contrary, the effect of
Ld-derive
d EVs, LPG, or SLA
also has an inhibitory effect on endogenous miR-146a levels in he-
patocytes as observed in Huh7 cells (FigS7D,E,andG). Taken together,
thedatasuggestthattheeffectof miR-146a rather than the Ld-derived
factors, as part of infected cell secreted EVs, has the potential to
change the IL-10 level in recipient na
¨
ıve macrophage cells and is
essential for m acrophage M2 po larizati on. Thus, we conclude that
the EV-associated miR-146a of Ld-infected cell EVs, have an anti-
inflammatory effect on na
¨
ıve macrophage cells and is associated
with an increase in IL-10 production in EV-treated cells, whereas the
LPS responsiveness as well as pro-inflammatory cytokine expression
level remain reduced in miR-146a–enriched RAW264.7 cells.
HuR is required for miR-146a–mediated IL-10 induction
From the data discussed so far, it has become more likely that miR-
146a control the IL-10 level in macrophage cells. How does miR-146a
ensure a high IL-10 expression in treated macrophage to polarize
them to have an anti-inflammatory response pathway—a prelude to
the infection niche establishment? HuR is an important post-
transcriptional gene regulator in mammalian macrophage and is
essential for activation and expression of proinflammatory cyto-
kines in macrophage exposed to LPS. Activated macrophage also
showed increased expression of HuR with LPS treatment time
(Goswami et al, 2020). Interestingly, expression of HA-HuR also
enhances the expression of pro-inflammatory cytokines in un-
treated macrophages and could get the cells to the activated state
(Fig 7A and B, Goswami et al, 2020). Therefore, to have the strong
anti-inflammatory effect of miR-146a, the HuR-mediated proin-
flammatory effect should be neutralized or countered by the
transferred miR-146a from the infected cells. Leishmania could
down-regulate HuR in mammalian macrophage cells (Fig S5H).
However, it is possible that the extracellular miR-146a released by
the infected cells could bring down the effect of HuR in noninfected
cells present in the infection niche to ensure an overall anti-
inflammatory pathway prevalent in all i mmune cells present
there. miR-146a–mediated down-regulation of HuR has been re-
ported previously (Cheng et al, 2013). Does miR-146a counter the
action of the HuR to stop inflammatory responses in macrophage
cells? In miR-146a–containing EV-treated cells, we have noted that
the expression of HuR could not enhance the IL-1β or TNF-α ex-
pression in RAW264.7 cells. Surprisingly, an increase in IL-10 ex-
pression in HuR expressing cells was detected when treated with
miR-146a–containing EVs (Fig 7C and D). Inversely, siHuR treatment
causes a reduction in IL-10 expression in cells treated with miR-
146a–containing EVs (Fig 7E and F). However, miR-146a mimic
decreases HuR levels as well as HuR induced increase in TNF-α and
IL-1β expression, whereas IL-10 level increases in presence of HA-
HuR when miR-146a is also expressed in RAW264.7 cells (Fig
7G–K).
How does HuR ensure the high IL-10 level? It is known that miR-21
negatively regulates IL-10 (Wang et al, 2017) and HuR is also known
to interact and sponge out miR-21 to inactivate it in mammalian
cells (Poria et al, 2016). We have noted reduction in miR-21 level in
HA-HuR–expressing cells, whereas siHuR treatment prevents miR-
21 lowering. We have also noted increased miR-21 export in HuR-
expressing cells that suggests HuR-mediated export of miR-21 that
helps the IL-10 expression (Fig 7L and M). Thus, HuR by up-
regulating IL-10 level also ensures the strong anti-inflammatory
polarization in miR-146a–containing EV-treated cells. Interestingly,
HuR eventually gets decreased in cells expressing high levels of
miR-146a to possibly balance the HuR-mediated stabilization of
cytokine mRNAs such as TNF-α and IL-1β possibly to restrict excess
independent experiments) and Pre-miR-122 (E, right panel , P = 0.0279, n = 4 independent experiments) were measured by qRT-PCR in Huh7 cells after pCIneo and pmiR-146a
expression in hepatocytes. U6 was used as endogenous control for miRNA normalization. pCIneo transfected cells was used for normalization of pmiR-146a transfected cells. For
Pre-miR-122 level, GAPDH was used as endogenous control. (F, G, H) Effect of EV treatment on Dicer1 level in na
¨
ıve hepatocyt es. (F) Level of Dicer1 was measured by Western blot in
control and Ld-infected cell–EV-treated Huh7 cells. β-Actin was used as loading control (F). (G) Dicer1 level was also measured in Huh7 treated with EVs derived from pCIneo and
pmiR-146a transfected RAW264.7 cells. β-actin was used as loading control. (H) Effect of pmiR-146a expression on Dicer1 in Huh7 hepatocytes was also determined. β-Actin was used
as loading control. (I) Effect of pmiR-146a overexpression on signalling component proteins in hepatocytes. Levels of HuR, P-ERK1/2, P-p38 were determined by Western blot
analysis in pCIneo and pmiR-146a transfected Huh7 cells. β-Actin was used as loading control. (J, K) Effect of NHA-Dicer1 expression on Dicer1 associated precursor miRNA level in
cells expressing pmiR-146a. (J) Average Ct value of NHA-Dicer1 associated Pre-miR-146a (J, left panel, P = 0.0005, n = 3 independent experiments, unpaired t test) and Pre-miR-122 level
(J, right panel, P = 0.2630, n = 3 independent experiments, unpaired t test) were determined by Real-time PCR after HA-Dicer1 was immunoprecipitated from pCIneo and pmiR-146a
transfected Huh7. (K) NHA-Dicer1wasimmunoprecipitatedusinganti-HAantibodyandwasdetected in immunoprecipitated and input samples using anti-HA antibody. HC, heavy
chain. Data information: In all experimental data, error bars are represented as mean ± SEM, ns, nonsignificant, *P <0.05,**P < 0.01, ***P < 0.001, respectively. P-values were calculated
by two-tailed paired t test in most of the experiments unless mentioned otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 11 of 23
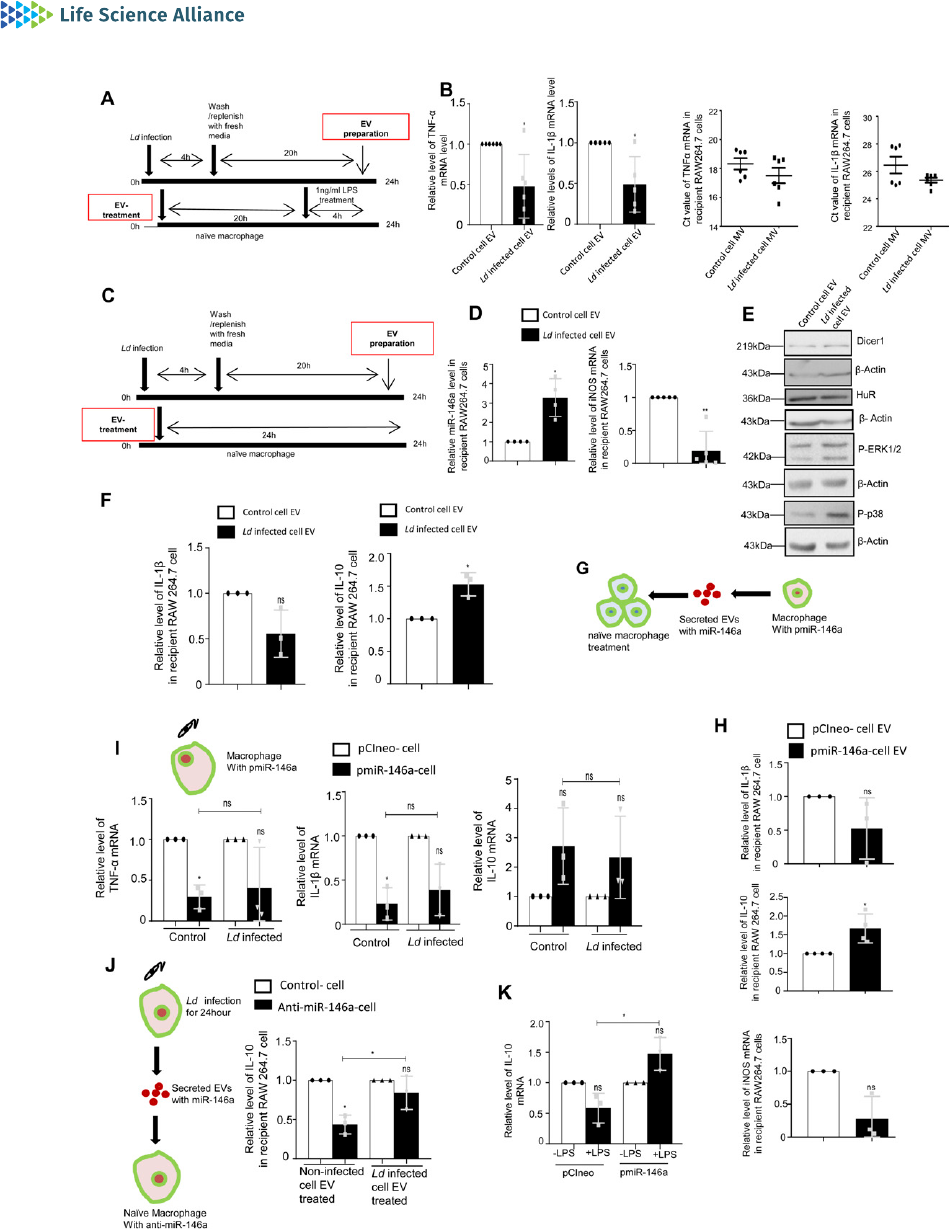
Figure 6. Extracellular vesicle (EV)–mediated transfer of miR-146a from infected macrophage cells promotes anti-inflammatory response in na
¨
ıve recipient macrophag es.
(A, B) Effect of EV treatment on LPS induced activation of recipient macrophages. (A) A schematic representation of the experiment has been shown in the left. (B) Relative
cellular levels of TNF-α (B, left panel, P = 0.0224, n = 6 independent experiments), IL-1β (B, middle left panel, P = 0.0280, n = 5 independent experiments) mRNAs were measured
by qRT-PCR from Infected cell–EV-treated macrophages after LPS treatment. TNF-α and IL-1β (B, middle right, P = 0.2487, n = 6 independent experiments, unpaired t test and
right panel, P = 0.1133, n = 6 independent experiments, unpaired t test, respectively) were measured by qRT-PCR from infected cell derived Microvesicle (MV)–treated
macrophages followed by 4 h of LPS (1 ng/ml) treatment. 18s rRNA was used as endogenous control. Values in the noninfected cell EV treated set were used asunits.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 12 of 23

anti-inflammatory response in miR-146a expressing cells. There-
fore, it is the balanced expression of HuR and miR-146a that de-
termine the polarization status of the macrophage. In miR-146a
compromised situation, expression of HuR promotes M1 polariza-
tion (Goswami et al, 2020), while HuR-mediated miR-21 export
contributes in increased IL-10 expression. High IL-10, in the pres-
ence of miR-146a, possibly down-regulates HuR protein level to
ensure the subdued expression of proinflammatory cytokines and
expression of IL-10 itself as a feedback effect (Rajasingh et al, 2006).
miR-146a acts as a balancer of IL-10 production and Ld infection
Does miR-146a uptake via EV affect the Ld infection process? We
have measured the number of parasites internalized in the treated
macrophage after Ld-infected cell EVs or miR-146a–containing EVs
treatment. We have performed quantification and noted reduced
entry of Ld after the miR-146a positive or infected cell EV treatment
(Fig 8A–D). Lower expression level of Leishmania amastigote–
specific gene amastin also confirmed reduced levels of Ld entry
after the miR-146a–containing EV or infected cell EV treatment of
RAW264.7 cells (Fig 8E –G ). miR-146a expression in RAW264.7 cells
also reduces the Ld infection process. We have used single cell
infection level examination to conclude on preferential reduction
of Ld internalization in cells expressing miR-146a (Fig 8H). Infection
level may be down-regulated due t o a problem i n signalling
pathways or downstream factors. We have noted an increased
P-p38 level in miR-146a–expressing macrophage that signifies the
existence of a possible counteractive pathway that usually gets
reduced in cells infected with Ld where a p38/MAPK down-
regulation has been noted (Fig 8M). The decreased Ld infection
could have also been attributed by a possible reduction in the
endocytosis process itself. However, in a latex bead internalization
assay, we did not document any reduction in latex bead inter-
nalization after miR-146a–containing EV or infected cell EV treat-
ment of RAW264.7 cells (Fig 8I–L).
Discussion
EVs are known for their role in intercellular communication. They
play an important role in immune response during any pathological
condition and also help to establish tumour microenvironment
(Thery et al, 2002). In disease condition, EVs either can help in
progression of the disease or in providing protection against the
disease. There are ma ny studies where it has been repor ted that
exosomes from Mycobacterium infected cells can trigger proin-
flammatory response in noninfected cells (Bhatnagar et al, 2007).
Leishmania parasitesreleaseexosomesasavehicletotransport
proteins to the host cell for immunosuppressive action (Silverman
et al, 2010). Leishmania protein G P63 was found to be t ransported
to neighbouring hepatocytes via secreted exosomes that target
Dicer1, a pr ocessor of pre-miRNAs, to down-regulate expression of
liver specific miR-122 (Ghosh et al, 2013). Leishmania parasite
secretes exosomes within sandfly midgut and thes e exosomes are
part of the sandfly inocula during bite which helps in patho-
genesis via overproduction of IL-17a in target cells (Atayde et al,
2015). Gioseffi et al (2020), in recent time, have reported Leish-
mania-infected EVs as a carrier of large number of Leishmanial
and host proteins (Gioseffi et al, 2020). Among the proteins
exported out, they have identi fied that a Leishmania homolog of
mammalian angiogenesis promoting factor, vasohibin is found to
induce endothelial cells to release angioge nesis promoting fac-
tors and thereby helping in promotion of lesion v ascularization
during infection.
miRNAs, being the regulator of expression of several cytokines,
are important players in determining the fate of immune cells and
in particular have a major role in buffering the immune activation
processes by regulating the expression of several cytokines directly
or indirectly (Lindsay, 2008; Testa et al, 2017). miR-146a and miR-155
are the two most important players that are explored for their
potential role as immune checkpoint regulators in the mammalian
system. Whereas miR-155 is a pro-inflammatory miRNA, miR-146a
(C,D,E,F)Effect of infected cell–derived EV treatment on recipient macrophages. (C) A schematic representation of the experiment has been shown (C). (D) Relative levels of
miR-146a and iNOS mRNA in recipient macrophage after 24 h of EV treatment were measured by qRT-PCR and miRNA and mRNA levels in noninfected cell EV-treated cell were
set as unit. U6 and GAPDH was used as endogenous control for miRNA and mRNA, respectively (D, left panel P = 0.0184, n = 4 independent experiments and right panel,
P = 0.0035, n = 5 independent experiments, respectively). (E) Levels of Dicer1, HuR, P-ERK1/2, and P-p38 were measured by Western blot analysis in infected cell–derived
EV-treated RAW264.7 cells. β-Actin was used as loading control. (F) Relative levels of IL-1β (F, left panel, P = 0.0967, n = 3 independent experiments) and IL-10 (F, right panel,
P = 0.0357, n = 3 independent experiments) were measured by qRT-PCR in recipient RAW264.7 cells after 24 h of EV treatment. GAPDH was used as endogenous control and
values for noninfected cell EV-treated cells were set as unit. (G, H) Effect of EV-mediated transfer of miR-146a on recipient macrophage. (G) A schematic diagram has been
given. (H) Relative levels of cytokine mRNAs; IL-1β,IL-10andiNOS(H,upper panel, P = 0.2131, n = 3 independent experiments, H, middle panel, P = 0.0406, n = 4 independent
experiments and H, lower panel, P = 0.0662, n = 3 independent experiments, respectively). GAPDH was used as endogenous control and values for pCIneo control EV-treated
cells were set as units. (I) Effect of Ld infection on cytokine mRNA levels in pmiR-146a overexpressed macrophages. A schematic diagram of the experiment has been shown
(I, upper panel). Relative cytokine mRNA level of TNF-α (I, left panel, P =0.0138,P =0.1740,P = 0.7287, n = 3 independent experiments), IL-1β (I, middle panel, P =0.0183,P =0.0684,
P = 0.4727, n = 3 independent experiments), and IL-10 (I, rightpanel, P =0.1491,P =0.2417,P = 0.7457, n = 3 independent experiments) were measured by qRT-PCR from pCIneo and
pmiR-146a transfected RAW264.7 cells after 24 h of Ld infection or no infection. GAPDH was used as endogenous control and values for pCIneo control cells were set as units
(I, unpaired t test was performed for comparison between pmiR-146a–
expressing noninfected control and infected set). (J
) Effect of control and infected cell derived-EV
treatment on recipient macrophages transfected with control or miR-146a inhibitor oligos. A schematic diagram has been given (J, left panel). Relative mRNA level of IL-10 was
measured by qRT-PCR from miR-146a inhibitor transfected and EV-treated RAW264.7 cells (J, right panel, paired t test, P = 0.0149,0.3208, unpaired t test was performed for
comparison between miR-146a inhibitor transfected control EV and infected cell EV-treated sets, P = 0.0456, n = 3 independent experiments, respectively). GAPDH was used as
endogenous control and values for negative control inhibitor transfected cells were set as units. (K) Effect of LPS treatment on pmiR-146a–expressing macrophages. Relative
level of IL-10 was measured by qRT-PCR from control or pmiR-146a–expressing cells with or without LPS exposure (1 ng/ml) (K. paired t test, P = 0.0984, 0.0921, unpaired t test
was performed for comparison between LPS-treated sets, P = 0.0132, n = 3 independent experiments). GAPDH was used as endogenous control and values for untreated control
cells were set as units. Data information: In all experimental data, error bars are represented as mean ± SEM, ns, nonsignificant, *P <0.05,**P < 0.01, respectively. P-values were
calculated by two-tailed paired t test in most of the experiments unless mentioned otherwise. Positions of molecular weight markers are marked and shown with the
respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 13 of 23

Figure 7. Crosstalk between miRNA-exporter HuR and miR-146a determines the fate of cytokine response in macrophage cells.
(A) LPS treatment (100 ng/ml) for different time points in RAW264.7 cells. The cell lysates were immunoblotted for HuR. β-Actin was used as loading control. (B) Real-
time PCR was performed for proinflammatory cytokine mRNAs (TNF-α and IL-1β) in pCIneo and pHA-HuR cells co-transfected with pre-miR control mimic. GAPDH was
used for normalization (n = 5 independent experiments; P-values = 0.2048, 0.0467, respectively). Va lues observed in pCIneo transfected cells were considered as units for
normalization of values obtained with pHA-HuR transfected cells. (C, D) Extracellular vesicles (EVs) isolated from pmiR-146a transfected RAW264.7 cells were treated to
recipient RAW264.7 cells expressing pCIneo or pHA-HuR. (C) Recipient cells treated with pmiR-146a–expressing cell EVs were immunoblotted for HA and HuR. β-Actin was
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 14 of 23

acts oppositely to balance the immune activation process (Testa
et al, 2017). In the infection context, the pathogens need to target
the miRNA pathways to control the inflammation level. The Ld
parasite has shown previously to control expression of miRNAs
in a negative manner in infected cells. By down-regulating HRS
and depolarizing mitochondria in the host, Ld affects the miRNA
recycling process to enhance the miRNA content of the infected
cells. However, the accumulated miRNAs are largely non-functional
as they were found to be accumulated with endosomal fraction and
they fail to recycle for new round of target repression on ER attached
polysomes (Chakrabarty & Bhattacharyya, 2017; Bose et al, 2020). The
miR-146a that also increases with Ld infection gets exported to
neighbouring cells. The miR-122 is another miRNA in hepatocytes that
Ld needs to control to ensure lower cholesterol biogenesis in the
liver cells essential for the parasite survival. Ld, by targeting Dicer1 in
hepatocytes, control the expression of miR-122 in infected liver tissue
(Ghosh et al, 2013; Chakrabarty & Bhattacharyya, 2017). Our results
show that miR-146a, transferred from infected macrophage cells,
also has a negative role to play on miR-122 expression in mouse liver.
Reciprocally, miR-122 can get transferred from activated or stressed
liver cells to resident macrophage to get them activated (Ghosh et al,
2015; Mukherjee et al, 2016). The parasite uses a secondary mech-
anism of lowering the miR-122 in liver cells to ensure reduced trans-
mission of the proinflammatory miR-122 to macrophages to prevent the
expression of inflammatory cytokines there. EV-mediated crosstalk
between the macrophages and hepatocytes fosters an environment of
cell-to-cell communication to take place. However, in the liver, it is
known that the EVs released from LPS-treated THP-1 macrophages
could stimulate the proliferation of hepatic stellate cells, whereras
miR-103-3p present in the EVs isolated f rom macrophages could
get transferred to the hepatic stellate cells to promote its growth
(Chen et al, 2020).
Mitochondrial activity is found to be a determining factor for
intercellular transfer of miRNAs. Does miR-122 affect mitochondrial
function? miR-122 is known to regulate mitochondrial metabolic
function. Cationic amino acid transporter gene CAT-1 level de-
creases, whereras PPARGC1A (PGC-1α) and succinate dehydroge-
nase subunit A and B level increases in HCC cells expressing
miR-122. PGC-1α is the regulator of mitochondrial biogenesis and it
is also involved in energy metabolism. Succinate dehydrogenase
(SDH) is the enzyme complex located in inner mitochondrial
membrane and is involved in both TCA cycle and electron transport
chain. PGC-1α and succinate dehydrogenase both are found to be
the putative secondary targets of miR-122 (Burchard et al, 2010).
Mitochondrial depolarization as well as ATP content are found to be
non-responsive to miR-122 levels in macrophage cells.
How mitochondria affects the EV entry in the recipient cells? We
have found how the mitochond ria-ER tethering and mitochondria–
endosome interaction play a key role in miRNA internalization process
which also affect miRNA turnover in mammalian cells (Chakrabarty &
Bhattacharyya, 2017). Any factor that can influence these interactions
will also modify organellar dynamics in mammalian cells to eventually
affect the EV-internalization. Increased expression of Ucp2 due to Ld
infection or exogeneous expression of FH-Ucp2 is associated with
mitochondrial depolarization and changed mitochond rial dynamics
that resulted in defective miRNA compartmentalization. Importantly
Mfn2 loss in Ld-infectedcellsorMfn2nullconditioninMfn2−/− MEF
cells are also associated with miRNA compartme ntalization defect
(Chakrabarty & Bhattacharyya, 2017). Therefore, microRNA compart-
mentalization or defective EV uptake possibly directly gets affected
by the change in mitochondrial dynamics rather than changed
membrane potential or ATP level. p-Triflouromethoxyphenylhydrazone
(FCCP) is an uncoupler of mitochond
rial membrane potential but does
not affect cellular ATP content whereas oligomycin inhibit F0F1-ATPase
used as loading control. (D) Real-time PCR was performed for cytokine mRNAs (TNF-α, IL-1β, and IL-10) in pCIneo and pHA-HuR transfected recipient cells treated with
pmiR-146a–EVs. GAPDH was used for normalization (n = 3 independent experiments; P-values: 0.3952, 0.0134, and 0.0352, respectively). Values obtained with pCIneo
transfected and EV-treated cells were considered as units. (E, F) EVs were isolated from pmiR-146a transfected RAW264.7 cells and used to treat the recipient RAW264.7
cells depleted of HuR (siHuR transfected). (E) Recipient cells treated with pmiR-146a–EVs were immunoblotted for HuR to check for proper silencing and β-Actin was
used as loading control (E). (F) Real-time PCR was performed for cytokine mRNAs (TNF-α, IL-1β and IL-10) in siCon or siHuR-containing recipient cells treated with pmiR-
146a–EVs, GAPDH was used for normalization (n = 3 independent experiments; P-values: 0.0004, 0.0015, 0.2641, respectively) (F). In this particular Experiment, siCon
transfected and EV-treated cells was used for normalization of values noted in siHuR-containing cells. (G, H, I, J, K) Effect of pHA-HuR expression in RAW264.7 cells
transfected with pre-miR control mimic or pre-miR-146a mimic. Transfection was performed either wit h pCIneo (control plasmid) or pHA-HuR. (G) Immunoblotting of the
cell lysates for HuR using β-Actin as loading control (G). (H) Immunoblotting of the respective cell lysates for HA to check for proper overexpression of HA-HuR, β-Actin
was used as loading control. Real-time PCR was performed for TNF-α, where GAPDH has served as control. (I) Values obtained for pCIneo and pre-miR control mimic co-
transfected sets were taken as units (n ≥ 5 independent experiments; P-values: 0.2048, 0.8587, 0.8620, respectively). (J) Real-time PCR was performed for IL-1β, GAPDH
served as control. Values obtained for pCIneo and pre-miR control mimic co-transfected sets were taken as units (n ≥ 5 independent experiments; P-values: 0.0467,
0.3453, 0.0375, respectively). (K) Real-time PCR was performed for IL-10, where GAPDH has served as control. Values obtained for pCIneo and pre-miR control mimic co-
transfected sets were taken as units (n ≥ 4 independent experiments; P-values: 0.0239, 0.2710, 0.0267, respectively). (L, M) Relative level of miR-21 in HA-HuR expressing
RAW264.7 cells. Real-time PCR for miR-21 level in RAW264.7 cells expressing pHA-HuR and transfected with pre-miR-146a mimic. Values obtained for pCIneo and pre-miR
mimic co-transfected sets were taken as units. U6 snRNA served as control (n = 3 independent experiments; P-value = 0.0015) (L, left panel). Real-time PCR for miR-21
level in recipient RAW264.7 cells depleted of HuR which were treate d with miR-146a–containing EVs. Values obtained for siCon and miR-146a–containing EV-treated sets
were taken as units. U6 snRNA served as control (n = 3 independent experiments; P-value = 0.5259) (L, middle panel). Real-time PCR of EV-associated miR-21 level from EVs
of RAW264.7 cells expressing or not expressing pHA-HuR. EV marker protein Flotillin-1 was used for normalization of miR-21 level (n = 3 independent experiments; P-
value = 0.4313) (L, right panel). Values obtained for pCIneo transfected cell–derived EVs was considered as unit. (M) Immunoblotting of the cell lysates with HA using β-Actin
as loading control in pCIneo or pHA-HuR transfected RAW264.7 cells.
(N) The
proposed model depicts the role of EV-associated miRNAs in Ld infection. The left half shows
that Ld infection triggers EV-mediated secretion of miR-146a from macrophage which when transfers to na
¨
ıve macrophage polarizes it toward M2 phenotype because of
its anti-inflammatory role whereas the right half represents what happens when miR-146a get delivered to na
¨
ıve hepatocyte via EV. miR-146a reduces miR-122 level in
hepatocytes. If not restricted, miR-22 as part of hepatocyte secreted EVs polarizes naive macrophage to M1 phenotype because of its proinflammatory role. Data
information: In all experimental data, error bars are represented as mean ± SEM,*P < 0.05; **P < 0.01; ***P < 0.001. P-values were calculated by two-tailed paired t test in
most of the experiments unless mentioned otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Source data are available for this figure.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 15 of 23

Figure 8. Effect of extracellular vesicle (EV)–associated miR-146a treatment on Ld infection.
(A, B, C, D) Infected cell–derived EV treatment decreases Ld infection in recipient cells. (A) Parasites were labelled with CFSE dye and infection was given for 6 h after 24 h
of control and infected cell–derived EV treatment (A, upper and lower panel, respectively). Parasite infection was determined by counting the CFSE positive structures
inside cells. Scale bar 10 μm. (B) Percentage of infected cells was calculated for both control cell EV and infected cell EV-treated RAW264.7 cells (B, P = 0.0064, unpaired
t test, n ≥ 16 number of fields). (C) Parasites were labelled with CFSE dye and infection was given for 6 h after 24 h of pCIneo and pmiR-146a–transfected cell–derived EV
treatment. Percentage of infected cells was calculated for both pCIneo and pmiR-146a transfected cell–derived EV-treated cells (C, P = 0.0208, unpaired t test, n ≥ 18
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 16 of 23

and prevents ATP production without affecting mitochondrial mem-
brane potential (Chakrabarty & Bhattacharyya, 2017). We found oli-
gomycin treatment could not inhibit EV-mediated internalization of
miR-122 and there was no change in miR-122 uptake in 3-h FCCP
treated cells. Thus, it is not the mitochondrial depolarization or ATP
level, but is the change in mitochondrial dynamics and its interaction
with endosomes or ER in Mfn2 KO or FH-Ucp2–expressing or Ld-
infected cells (Chakrabarty & Bhattacharyya, 2017) that probably
hampers endocytosis of miR-122–containing EVs in recipient cells.
MicroRNAs are the small noncoding RNAs that are involved in
post-transcriptional and translational regulation of various genes.
Thus, altered expression of these miRNAs is one of the main
reasons for various pathogenic conditions (Bartel, 2018). Earlier
reports suggested up-regulation of miR-146a/b during Salmonella
infection (Ordas et al, 2013). Up-regulation of miR-155 and miR-
146a/b and down-regulation of miR-20a, miR-191, and miR-378 in
Mycobacterium avium–infected condition were also reported
(O’ Conne ll et al, 2012; Curtale et al, 2019 ). miR-155 favours the
pro-inflammatory environment, whereas miR-146a favours the
anti-inflammatory environment (Lindsay, 2008). Recently miR-
146a–mediated M2 polarization during L. donovani (Ld) infection
has been reported. The investigation revealed that L. donovani (Ld)
infection up-regulates miR-146a expression which favoured par-
asite survivability in hosts by maintaining the anti-inflammatory
environment. Inhibition of miR-146a lowered parasite burden in
infected Balb/C mice as well as anti-inflammatory environment (M2
phenotype). Interestingly, this up-regulation of miR-146a during Ld
infection is regulated by Super Enhancer components like BET
bromodomain 4 (BRD4) and P300 (Das et al, 2021).
The polarization of macrophage is contributed by several factors
including specific activation of signalling component that favours
either the TLR4-p38/MAPK-NF-kB–mediated activation of proin-
flammatory cytokine expression or it may be an inhibitory circuit
involving ERK1/2–dependent inactivation of the proinflammatory
cytokines with con comitant increase in expression of anti-
inflammatory cytokine like IL-10 (Mukherjee et al, 2021). The par -
asite s eems to use the infected cell EVs packed with miR-146a to
transmit the anti-i nflammatory signals to the na ive neighbouring
macrophage to polarize them to the M2 stage. It is not known
whether the polarization is reversible, but subsequent treatment
of miR-146a overexpressed cell s with LPS suggest the refractory
nature of the recipient macrophage to inflammatory response.
Thus, miR-146a–containing EVs could be considered as a good
immune modulator and use of that could be important to control
the inflammation in different physiological contexts like in pre-
vention of viral infection related cytokine storm (Ar isan et al, 2020
)
or
inflammation associated with tumour (Rupaimoole et al, 2016).
The cross communication of miR-146a–containing EVs to other
noninfected tissue is another important aspect. Do EVs with miR-
146a get transferred via the bloodstream to the spleen and do they
have any effect on subsequent establishment of infection and
T-cell polarization observed in the spleen of infected animals?
These are important questions for futur e exploration.
In our experimental system, the effect of Leishmanial secretory
proteins on the M2 state of macrophages seems to be marginal as
the cytokine pattern of recipient macrophages treated with Ld
promastigote culture–derived EVs showed decreased level of IL-10
and IL-1β as well. Thus, these results clearly explain that the ob-
served M2 state of the na
¨
ıve macrophages treated with infected
cell–derived EVs is due to EV-mediated transfer of miR-146a and
possibly not by Leishmania specific factors. The results obtained
from the SLA or LPG or parasite-derived EV-treated macrophages
and hepatocytes suggest Leishmania derived factors are not pri-
marily responsible also for miR-122 and miR-146a up-regulation in
respective cell types (Fig 7N).
Although miR-122 can up-regulate IL-1β mRNA, we could not
detect an increase in mature IL-1β by ELISA in miR-122 expressing
cells. Thus, the transcriptional surge of IL-1β mRNA due to miR-122
expression is not associated with increase detectable in the protein
level. It is possible that the excess IL-1β mRNA produced in the
presence of miR-122 may get transferred to other immune cells
where they may elicit a proinflammatory response. Otherwise, the
inflammatory response observed in miR-122 expressing cells is
manifested primarily by TNF-α rather than IL-1β.
HuR is a RNA binding protein with immense functional diversity.
HuR is known to stabilize the mRNAs with AU-rich elements in their
39 UTR and thus have a role in the inflammation process as most of
the proinflammatory cytokines such as TNF-α or IL-1 β bear the AU-
rich elements in their 39 UTR (Srikantan & Gorospe, 2012). In this
report, we have detected a non-canonical role of HuR in stabili-
zation of IL-10 mRNA. The anti-inflammatory IL-10 mRNA is regu-
lated negatively by miR-21 (De Melo et al, 2021), the suppression of
number of fields). (D) Parasites were labelled with CFSE dye and infection was given for 24 h to pCIneo and pmiR-146a–transfected cell. Percentage of infected cells was
calculated for both pCIneo and pmiR-146a transfected cells (P = 0.0401, unpaired t test, n ≥ 16 number of fields). (E) RAW264.7 cells were infected with Ld for 24 h and
infection was measured in terms of amastin mRNA level by qRT-PCR (E, P = 0.2213, n = 4 independent experiments). (F) Relative level of amastin mRNA was determined by
qRT-PCR from control and infected cell–der ived EV-treated cells followed by 6 h infection (F, P = 0.0205, n = 4 independent experiments). (G) Relative level of amastin
mRNA from pCIneo and pmiR-146a transfected cells after 24 h of infection was determined by qRT-PCR (G, P = 0.0160, n = 4 independent experiments). GAPDH was used as
endogenous control. Values for uninfected cell or control EV-treated cell or pCIneo transfected cells were considered as units. (H) RAW264.7 cells were co-transfected
with DsRed and pmiR-146a and was infected with Ld for 24 h and infection level was visualized at individual cells (lower panel) and quantified (upper panel). Percent of
infection in pmiR-146a/DsRed expressing versus non-transfected Ld-infected cells were measured. Scale bar 10 μm. (n = 38, Number of fields). (I, J, K, L) Latex bead
phagocytosis after EV treatment. Red fluorescent bead (Fluorescent Red; Sigma-Aldrich) was diluted in medium and added to EV-treated RAW264.7 cells at 1:10 (cell:bead)
ratio for 6 h. Red fluorescent bead was visualized by a confocal microscope. (I, J) Phalloidin 488 was used for staining the cytoskeleton of cells (I). Scale bar 10 μm.
Percentage of beads phagocytosed in EV-treated macrophages was plotted and compared between control cell EV-treated and Ld-infected cell EV-treated cells (J,
unpaired t test, P = 0.0347, Number of fields, n ≥ 13 number of fields). (K, L) In panel (K) visualization of beads phagocytosed in EV-treated macrophages was monitored in
pCIneo transfected control cell EV-treated and pmiR-146a– expressing cell EV-treated cells. The quantification was completed and plotted in panel L (unpaired t test, P =
0.6761, Number of fields, n ≥ 13 number of fields). Scale bar = 10 μm. (M) Cellular levels of the major signalling pathway components in pmiR-146a and pCIneo expres sion
conditions were determined by Western blot. β-Actin was used as loading control. Data information: In all experimental data, error bars are represented as mean ± SEM,
ns, nonsignificant, *, ** represent P-value of <0.05, <0.01, respectively. P-values were calculated by two-tailed paired t test in most of the experiments unless mentioned
otherwise. Positions of molecular weight markers are marked and shown with the respective Western blots.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 17 of 23

miR-21 activity by HuR has been reported previously (Poria et al,
2016). In the context of miR-146a induced expression of IL-10, HuR
acts synergistically to remove miR-21 from the cell to boost the anti-
inflammatory IL-10 production in macrophages. Interestingly, in
the context of an anti-inflammatory environment the miRNA
antagonistic role of HuR predominate to ensure expression of
miRNA-repressed cytokine mRNAs, whereas in the proinflammatory
context, the mRNA ’s stabilizer role of HuR is important to ensure
proinflammatory cytokine expression there.
Materials and Methods
Cell culture, peritoneal macrophage isolation, and parasite
infections
Human hepatic Huh7, mouse hepatic HePa1-6, and Mfn2 wild-type
and knockout MEFs cells (WT/Mfn2
−/−
) were cultured in Dulbecco’s
Modified Eagle’sMedium(Gibco-BRL)and 10% FBS (heat-
inactivated foetal bovine serum). In case of RAW264.7 macro-
phage cells, RPMI-1640 medium (Gibco) with 2 mM L-glutamine and
0.5% β-mercaptoethanol along with 10% FBS was used. Primary
murine peritoneal macrophages were isolated from BALB/c mice
subjected to intraperitoneal injection of 1 ml of 4% starch solution.
Cold 1× PBS was used to lavage or wash the peritoneal cavity to
isolate the peritoneal macrophages the following day which were
then pelleted and plated. These cells were also cultured in RPMI-
1640. EV isolation of Peritoneal Exudate Cells (PECs) was done from
a 60 mm plate. All experiments with PEC were carried out after 48 h
of isolation (Goswami et al, 2020 ). For macrophage or Huh7 acti-
vation, LPS dose of 1 or 100 or 500 ng/ml was used for different time
points depending on the experiments. RAW264.7 and Huh7 cells
were stimulated with LPS from Escherichia coli O111:B4 (Calbio-
chem). For EV-free growth medium, 10% EV-depleted FBS (made by
ultracentrifugation of FBS at 1,000,00g for 4 h) was added to DMEM
or RPMI-1640 for respective cell types.
Animal experiments
BALB/c male mice (4–6 wk) were divided into four groups (three
animals each) of either uninfected or infected and treated or
untreated with EVs. Two groups (six animals) were infected with
1×10
7
promastigotes by intracardiac puncture and maintained for
30 d. On the 30
th
day, EVs were injected into six animals (three
infected and three uninfected with parasites), whereas 1× PBS was
injected in the remaining six animals (three infected and three
uninfected). For EV isolation, HePa1-6 cells were transfected with
miR-122–expressing plasmid and ~5 × 10
9
EVs (measured by Nano-
particle Tracker Nanosight NS300) isolated from these transfected
cells were suspended in 1× PBS and injected into the tail vein. The
animals were euthanized on the 31
st
day for Kupffer cell isolation.
All the experiments were carried out in accordance with the
National Regulatory Guidelines issued by the Committee for the
Purpose of Supervision of Experiments on Animals, Ministry of
Environment and Forest, Govt. of India. The animal experiments
were performed in agreement with the Institutional Animal Ethics
Committee. The BALB/c mice were kept under controlled conditions
(temperature 23 ± 2°C, 12 h/12 h light/dark cycle) in individually
ventilated cages.
Kupffer cell isolation was done from control and experimental
group of mice. The animals were anaesthetized, and livers were
perfused with HBSS via the hepatic portal vein with an incision in
the inferior vena cava until they turned bloodless and then
digested with a Liver Digest Medium. Livers were then excised,
minced, and filtered through a 70-μm cell strainer. The resultant
single cell suspension was centrifuged at 50g for 5 min to pellet
and store the hepatocytes. The supernatant was then collected and
loaded onto a Percoll gradient of 25% and 50%. It was centrifuged at
1,200g for 30 min at 4°C without brakes. The interphase containing
the Kupffer cells was collected and washed twice with PBS and the
pellet was stored. The Kupffer cell pellet and hepatocyte pellet were
subjected to RNA isolation by TriZol reagent.
For detection of purity of isolation, the Kupffer cells of all the four
groups were compared with the respective hepatocytes by quan-
tification of mRNA levels of C-type Lectin Domain Family 4, Member
F (Clec4f), a Kupffer Cell marker, and hepatocyte marker, Albumin.
The kinetoplast DNA levels were also quanti fi ed in the four groups
to detect the level of leishmanial infection.
Parasite culture and infection to macrophage cell line
L. donovani (Ld) strain AG83 (MAOM/IN/83/AG83) was obtained
from a Indian Kala-azar patient and was maintained in golden
hamsters (Chakrabarty & Bhattacharyya, 2017). Amastigotes were
obtained from infected hamster spleen and transformed into
promastigotes. Promastigotes were maintained in M199 medium
(Gibco) supplemented with 10% FBS (Gibco) and 1% Pen-Strep
(Gibco) in 22°C. RAW264.7 cells or PEC were infected with station-
ary phase Ld promastigotes of second to fourth passage at a ratio of
1:10 (cell: Ld) for 6, 16, or 24 h depending upon the experiment
(Goswami et al, 2020).
Plasmid constructs and transfection
pCIneo, precursor miR-146a–overexpressing plasmid (pmiR-146a),
precursor miR-122–overexpressing plasmid (pmiR-122), and HA-HuR
plasmid(pHA-HuR) were transfected using Fugene HD (Promega) for
RAW264.7 cells as described previously (Goswami et al, 2020). For
Huh7 cells, all transfections of plasmids were performed using
Lipofectamine 2000 reagent (Life Technologies) according to the
manufacturer’s protocol. For miRNA inhibitor (anti-miR-146a) ex-
periments, 30 pmol per well (30 nM) was transfected using RNA imax
reagent in RAW264.7 cells according to manufacturer’s protocol.
Transfection of Negative control inhibitor was used as controls. For
co-transfection of plasmid and pre-miR mimic, Lipofectamine 2000
(Invitrogen) was used. 50 pmoles of pre-miR mimic were trans-
fected per well of a 12 well plate (50 nM). siRNA transfection was
performed using RNAi max (Invitrogen) following the manufac-
turer’s instructions. RAW264.7 cells were transfected with 50 pmoles
of siRNA per well of a 12-well plate (50 nM). siRL was used as siCon
for the experiments. EV treatment was given to the recipient cells
48 h after siRNA transfection and the cells were harvested 24 h after
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 18 of 23

EV treatment. Donor RAW264.7 cells were transfected with 2 μg
pmiR-146a plasmid per well of a six-well plate using Fugene HD.
EV isolation and characterization
RAW264.7 cells were plated in a 90 mm plate and given infection
with stationary phase parasite at about 80% confluency and kept
for 6 h. After 6 h of infection, the medium was discarded and cells
were washed with PBS to remove extracellular unattached para-
sites and cells were replenished with fresh RPMI-1640 medium
supplemented with 10% EV depleted FBS and 1% Pen-Strep for 20 h.
Total infection time was 24 h before EV isolation. After 24 h, the
supernatant was collected for EV isolation. Briefly, the supernatant
first centrifuged at 3,000g for 15 min at 4°C followed by 30 min
centrifugation at 10,000g at 4°C. For all EV isolation experiments,
supernatant was passed through a 0.22-μm filter unit followed by
ultracentrifugati on on a 30% sucrose cushion at 1,00,000g for 70 min.
The supernatant was discarded leaving a medium layer of interface-
containing EVs behind, which was then washed with 1× PBS at 1,00,000g
for 70 min again. The pellet then was resuspended in PBS for NTA or 1×
passive lysis buffer (PLB) for RNA or protein precipitation (Promega).
For EV-associated RNA and protein, the pellet was resuspended in PLB.
One-third was used for total RNA isolation using TriZzol LS reagent
(Invitrogen) following the manufacturer’s protocol. Two-thirds of the
PLB sample was used for protein precipitation using methanol pre-
cipitation. In brief, for 200 μlofPLBsample,640μl of sterile water,
480 μl of methanol (Merck), and 160 μl of chloroform (Sigma-
Aldrich) w ere added and vortexed well followed by centrifugation
at 20,000g for 5 min at room temperature. Then the supernatant
was discarded and 300 μl o f methanol was added. The mixture
was vortexed well and again centr ifuged for 30 min at 20,000g 4°C.
The supernatant was discarded. The pellet was air drie d for 1 0 min
and then dissolved in 1× SDS Dye and heated at 95°C before run on
aSDS–PAGE.
For experiments with EV treatment, culture supernatant first
centrifuged at 3,000g for 15 min at 4°C followed by 30 min cen-
trifugation at 10,000g and filtered pass through 0.22 μm following
ultracentrifugation at 1,00,000g for 70 min. The pellet was then
resuspended in RPMI-1640 medium supplemented with 10% EV
depleted FBS and 1% Pen-strep for further use.
For nanoparticle tracking analysis (NTA), the control and infected
RAW264.7 cell–derived EV pellet was resuspended in 300 μlof
sterile PBS and resuspended well. Then, diluted 10-folds in PBS and
1 ml of diluted sample was used for NTA (Nanosight NS300).
EV treatment of recipient cells
After EV isolation, the pellet was resuspended in fresh RPMI-1640
supplemented with 10% EV–depleted FBS and 1% Pen-Strep fol-
lowed by filtration through a 0.22-μ
m filter
unit. The EVs were then
added to the recipient macrophage or hepatic cell line for 24 h.
Post-LPS treatment was given at a dose of 1 ng/ml for 4 h after 20 h
of EV treatment to macrophages. For treatment, EVs isolated from
8×10
6
cells (one 90 mm plate) were added to 2.4 × 10
5
recipient cells
(per well of a 12 well plate). For estimation of parasite infection after
EV treatment, parasites were added to EV-treated cells for 6 h.
Optiprep density gradient ultracentrifugation
For cell fractionation using 3–30% iodixanol gradient (Optiprep
gradient) 1.6 × 10
7
cells were used as described earlier (Mukherjee
et al, 2016). Optiprep (Sigma-Aldrich) solution was used to prepare
3–30% gradient. In brief, the cell pellet was homogenized in a
buffer with glass Dounce homogenizer. The lysate was centri-
fuged at 1,00 0g twice and the supernatant was loaded on the
gradient and centrifuged at 36,000 rp m in a Beckman-Coulter
SW60Ti rotor for 5 h. Approximately 400 μl of 10 fractions were
collected for RNA and protein.
Total RNA isolation and quantification of miRNA and mRNA
Total RNA extract from experimental samples using TriZol reagent
(Invitrogen) for cell and TriZol LS (Invitrogen) for Optiprep/
subcellular fractio n according t o manufacturer ’sprotocol.All
miRNA and mRNA quantifications were performed as described
previously ( Basu & Bhattacharyya, 2014; Mukherjee et al, 2016). In
brief, miRNA estimation was performed with 100 ng of RNA for cellular
and EV samples using specific primers for human miR-146a, mouse
miR-155, human miR-122, human miR-21, human miR-16, miR-125b,
and U6 snRNA was used as endogenous control. For the assay, one-
third of the reverse transcription mix was subjected to PCR ampli-
fication with TaqMan Universal Master Mix No AmpErase (Applied
Biosystem) and respective TaqMan assay reagents for target miRNAs.
Samples were analysed in triplicates for each biological replicates.
The comparative C
t
method which involved normalization by U6
snRNA was used for quantification (Mukherjee et al, 2016).
For mRNA, 200 ng of total RNA was used for estimation based on
SYBR Green based real-time cDNA estimation using specific primer
for target genes. The comparative C
t
method which involved nor-
malization by GAPDH or 18s rRNA was used for relative quantifi-
cation of mRNA (Goswami et al, 2020). The details of Primers, miRNA
assays are provided as part of Tables S4 and S5. Information on
expression plasmids, siRNAs, miRNA-mimic and miRNA inhibitors
are part of Table S6.
Western blot
Western blot analyses were performed as described elsewhere
(Mazumder et al, 2013). Imaging of the blots was performed using an
UVP BioImager 600 system equipped with VisionWorksLife Science
software (UVP) V6.80. ImageJ software was used for quantification.
Details of all antibodies used for Wes tern blot and immuno fluo-
rescence experiments are summarized in Tab le S 7.
Immunofluorescence
For internalization, Ld parasites were stained with 1 μ M carboxy-
fluorescein succinimidyl ester (CFSE) dye (green) for 30 min at 22°C
followed by PBS wash thrice and then resuspended in medium
before adding to the cell. Cells were fixed with 4% PFA for 20 min
after three PBS wash. Nuclei were stained with DAPI. For calculating
the percentage of infected cells, a minimum number of 100 cells
were counted. Cells were observed under the Zeiss LSM800 con-
focal microscope.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 19 of 23

Polysome isolation
For polysome isolation ~8 × 10
6
cells were used as starting material.
After 24 h of infection, cells were scraped in PBS and pelleted down
at 600g for 5 min at 4°C. The cell pellet was collected and incubated
in 1× lysis buffer (10 mM Hepes, 25 mM KCL, 5 mM MgCl
2
, 1 mM DTT, 5
mM VRC, 1× PMSF, cycloheximide 100 μg/ml, 1% Triton X-100, and 1%
sodium deoxycholate) for 15 min at 4°C followed by centrifugation
at 3,000g for 10 min at 4°C. Then the supernatant was collected and
centrifuged at 20,000g for 10 min at 4°C. The supernatant was
collected and loaded on 30% sucrose cushion in gradient buffer
and centrifuged at 31,200 rpm for 1 h in 4°C in a SW61Ti rotor.
After centrifugation the non-polysome fractions were collected
andtherestofthesolutionwasmixedfollowedbycentrifugation
at 31,200 rpm for 30 min at 4°C in a SW61Ti rotor. T he pelleted
polysome was suspended in polysome buffer (10 mM Hepes,
25 mM KCL, 5 m M MgCl
2
, 1 mM DTT, 5 mM VRC, a nd 1× PMS F) and
kept for RNA isolation and Western blot (Ghoshal et al, 2021).
LPG extraction and treatment
LPG isolation was performed as mentioned earlier (Goswami et al,
2020). In brief, 2 × 10
8
stationary phase parasites (AG83) were used
for LPG extraction. The pellet was resuspended in 2 ml of Chlo-
roform: Methanol: Water mixture (1:2:0.5 vol/vol) followed by vor-
texing and sonication for 10 s thrice at 30 s interval and incubated in
mutarotator at room temperature for 2 h. Then the lysate was
centrifuged at 4,000g for 30 min at 4°C. The pellet was used for
extraction in 9% 1-Butanol in water. After vortex and sonication as
before, pellet was incubated for 1 h at room temperature in
mutarotator. Second extraction was done with the pellet in 9%
1-Butanol in water again after centrifugation at 4,000g for 30 min at
4°C. The supernatants of both extractions were pooled for ly-
ophilization. The lyophilized LPG was resuspended in 1 ml sterile
PBS before cell culture treatment. For treatment, 1:50 (cell: LPG)
dose was used for different time points.
SLA preparation
SLA was prepared from the stationary-phase promastigotes (~10
8
cells) as described earlier (Basu et al, 2005). Briefly, stationary
phase parasites were pelleted down at 3,000g for 10 min followed
by repeated cycles of freezing (−70°C) and thawing (37°C) followed
by 5 min incubation on ice. These cells were then further completely
lysed by sonication thrice for 30 s and centrifuged for 10,000g for
30 min at 4°C. The supernatant was used for protein estimation
using the Bradford assay. Macrophages or Huh7 were treated with
10 μg/ml SLA for 24 h.
Mass spectrometry
For proteomics sample preparation, exosomes from two 90 mm
were pooled and protein was precipitated with 100% chilled ace-
tone in −20°C overnight. The protein precipitated was then washed
with 80% chilled acetone followed by another wash with 40%
chilled acetone. The pellet was then air dried for 5–10 min to
remove excess acetone at room temperature and resuspended in
50 mM ammonium bicarbonate (AmBic) followed by sonication
thrice. 85 mM DTT was then added, and the sample was heated at
60°C for 1 h. Sample was then incubated in dark with 55 mM
Iodoacetamide (IAA) at RT for 30 min. Trypsin (1 μg/μl) digestion was
carried out to the sample for overnight at 37°C. The reaction was
stopped with 100% formic acid (5% of total volume) and snap frozen
until lyophilization. Lyophilized sample was used for proteomics
and run in Orbitrap analyzer.
Latex bead phagocytosis assay
Red fluorescent lab elled Latex bead (Fluorescent Red; Sigma-
Aldrich) was diluted in RPMI-1640 medium and added to mac-
rophages at a ratio of 1:10 (cell:bead) for 6 h. Cells were fixe d w ith
4% PFA as described earlier and mount in DAPI. Cell cytoskeleton
was s tained with Phalloidin 488. Red latex bead phagocytosis was
imaged in Zeiss LSM800 confocal microscope.
Cytokine measurement by ELISA
Protein level of TNF-α was measured from culture s upernatant
of RAW264.7 macrophages using sandwich ELISA Kit (BD Phar-
mingen) as per the manufacturer ’ s protocol. Protein l evel of IL-
1β was m easured from RAW264.7 cell lysate (1 mg/ml) instead of
cell culture supernatant using sandwich ELISA Kit (BD Phar-
mingen) as per the manufacturer’sprotocol.Cytokinelevelwere
determined by measuring the OD at 450 nm using a microtiter
plate reader.
Measurements of NO in cell culture supernatant
Nitric oxide (NO) was measured from RAW264.7 cell supernatant
using G reiss reagent. In brief, equal volume of sample was in-
cubated with eq ual volume of 1% sulphanilam ide for 15 min in
dark at room temperature followed by equal volume of 0.1%
NEDD (N-1-napththylethylenediamine dihydrochloride) was added
and incubated until colour developed. NO level was deter-
mined by measuring the OD at 550 nm using a microtiter plate
reader.
Flow cytometry analysis for mitochondrial depolarization
Approximately 0.4 × 10
6
RAW264.7 cells were scraped and washed in
PBS. Then the cells were incubated in 100 nM MitoTracker Red for
30 min in 37° C followed by washing with PBS to remove excess stain
and finally analysed in BD FACS Fortessa.
Cellular ATP-level measurement
Amount of ATP was determined using the ATP bioluminescent assay
Kit (Sigma-Aldrich)
. Approximately 1.5 × 10
6
RAW264.7 cells were
scraped and washed in PBS. Briefly, the cells were lysed and the
equal volume of lysate was added to equal volume of ATP assay mix
solution followed by rapid mixing and the amount of light produced
was determined by luminometer.
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 20 of 23

Immunoprecipitation
Immunoprecipitation was performed as described earlier with
minor modifications (Goswami et al, 2020). Briefly, protein G agarose
beads (Life Technologies) was used for exogeneous HA protein or
any other endogenous protein. Beads were washed twice with 1× IP
buffer (20 mM TRIS–HCL, pH 7.5, 150 mM KCL, 5 mM MgCl
2
, and 1 mM
DTT 1× EDTA free protease inhibitor cocktail) at 2,000g for 2 min at
4°C. Beads were then blocked with 5% BSA in lysis buffer for 1 h at
4°C in mutarotator followed by washing with IP buffer as mentioned
previously. Afte r blocking, requi red amount of antibody was
added to t he bead for a nother 4 h incubation at 4°C before lysate
addition. The final dilution of antibody was 1:100. Cells w ere
lysedinalysisbuffer(1×IPbufferwith0.5%TritonX-100and0.5%
of sodium deoxycholate) for 30 min in 4°C and sonicated thrice
for 10 s interval and 30 s incu bation in ice. Then l ysate was
collected after centrifugation at 16,000g for 10 min at 4°C and
was added to the bead-antibody mixture for overnight immu-
noprecipitation to occur at 4°C. Af ter the reaction, the lysate–
bead–antibody mixture was washed with 1× IP buffer thrice and
was resuspended i n 400 μl I P buffer. Equal volume was kept for
protein and RNA. For RNA, TriZol LS was added thrice of the
volume and for protein 5× SDS dye was added so that the final
concentration would be 1×.
Statistical analysis
All graphs and statistical significance were calculated using
GraphPad Prism 5.00 and 8.00 version (GraphPad). Experiments
were carried out for minimum three times to get P-value, unless
otherwise mentioned. Two-tailed paired and unpaired t tests were
used for determination of P-values. The sample size was chosen by
convenience and no exclusion criteria were used.
Ethics statement
Balb/C mice and Syrian golden hamsters w ere obtained from
CSIR-Indian Institute of Chemical Biology animal house facility.
All experiments were performed according to the national
regulatory guidelines stated by the Committee for the Purpose of
Supervision of Experiments on Animals, Ministry of Environment
and Forest, Govt. of India. All animal experiments were per-
formed with prior approval of the Institutional Animal Ethics
Committee.
Data Availability
All data are available in the supplementary Tables (Tables S1–S3).
This study includes no data deposited in external repositories.
Supplementary Information
Supplementary Information is available at https://doi.org/10.26508/lsa.
202101229.
Acknowledgements
We acknowledge Witold Filipowicz for different plasmid constructs. We also
acknowledge the support of Malini Sen, Shilpak Chatterjee , and Syamal Roy
for different reagents used in ELISA and ATP measurement assays. SN
Bhattacharyya and K Mukherjee acknowledge Council of Scientific and In-
dustrial Research (CSIR)-Indian Institute of Chemical Biology (IICB) for
infrastructural support. The work is supported by The Swarnajayanti Fel-
lowship (DST/SJF/LSA-03/2014-15) and CSIR funded Niche Creating Project
RNAMOD (MLP-139), Leishmaniasis (MLP-136), and the Indo-Swiss Bilateral
Project Grant from Department of Biotechnology, Govt of India (BT/IN/
Swiss/53/SNB/2018-19). K Mukherjee and SN Bhattacharyya both received
support from CSIR. SN Bhattacharyya also acknowledges support of National
Bioscience Award Fund from Department of Biotechnology, Government of
India. S Ganguly received support from University Grant Commission, India. B
Ghoshal, I Banerji, S Chakraborty, S Bhattacharjee, and A Goswami received
their support from CSIR, India. The funders had no role in study design,
data collection and analysis, decision to publish, or preparation of the
manuscript.
Author Contributions
S Ganguly: data curation, formal analysis, validation, investigation,
and methodology.
B Ghoshal: formal analysis, valida tion, investiga tion, and methodology.
I Banerji: data curation, formal analysis, validation, investigation,
and methodology.
S Bhattacharjee: validation and investigation.
S Chakraborty: validation and investigation.
A Goswami: validation and investigation.
K Mukherjee: conceptualization, formal analysis, supervision, vi-
sualization, methodology, and writing—original draft, review, and
editing.
SN Bhattacharyya: conceptualization, data curation, supervision,
visualization, methodology, project administration, and writing—original
draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
Arisan ED, Dart A, Grant GH, Arisan S, Cuhadaroglu S, Lange S, Uysal-Onganer
P (2020) The prediction of mirnas in sars-cov-2 genomes: Hsa-mir
databases identify 7 key mirs linked to host responses and virus
pathogenicity-related kegg pathways significant for comorbidities.
Viruses 12: 614. doi:10.3390/v12060614
Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M (2015)
Exosome secretion by the parasitic protozoan leishmania within the
sand fly midgut. Cell Rep 13: 957–967. doi:10.1016/j.celrep.2015.09.058
Bartel DP (2018) Metazoan micrornas. Cell 173: 20–51. doi:10.1016/
j.cell.2018.03.006
Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S (2005) Kinetoplastid
membrane protein-11 DNA vaccination induces complete protection
against both pentavalent antimonial-sensitive and -resistant strains
of leishmania donovani that correlates with inducible nitric oxide
synthase activity and il-4 generation: Evidence for mixed th1- and th2-
like responses in visceral leishma niasis. J Immunol 174: 7160–7171.
doi:10.4049/jimmunol.174.11.7160
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 21 of 23

Basu S, Bhattacharyya SN (2014) Insulin-like growth factor-1 prevents mir-122
production in neighbouring cells to curtail its intercellular transfer to
ensure proliferation of human hepatoma cells. Nucleic Acids Res 42:
7170–7185. doi:10.1093/nar/gku346
Beattie L, Peltan A, Maroof A, Kirby A, Brown N, Coles M, Smith DF, Kaye PM
(2010) Dynamic imaging of expe rimental leishmania donovani-
induced hepatic granulomas detects kupffer cell-restricted antigen
presentation to antigen-specific cd8 t cells. PLoS Pathog 6: e1000805.
doi:10.1371/journal.ppat.1000805
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released
from macrophages infected with intracellular pathogens stimulate a
proinflammatory response in vitro and in vivo. Blood 110: 3234–3244.
doi:10.1182/blood-2007-03-079152
Bode JG, Ehlting C, H
¨
aussinger D (2012) The macrophage response towards
lps and its control through the p38(mapk)-stat3 axis. Cell Signal 24:
1185–1194. doi:10.1016/j.cellsig.2012.01.018
Bose M, Chatterjee S, Chakrabarty Y, Barman B, Bhattacharyya SN (2020)
Retrograde trafficking of argonaute 2 acts as a rate-limiting step for
de novo mirnp formation on endoplasmic reticulum-attached
polysomes in mammalian cells. Life Sci Alliance 3: e201800161.
doi:10.26508/lsa.201800161
Burchard J, Zhang C, Liu AM, Poon RT, Lee NP, Wong KF, Sham PC, Lam BY,
Ferguson MD, Tokiwa G, et al (2010) Microrna-122 as a regulator of
mitochondrial metabolic gene network in hepatocellular carcinoma.
Mol Syst Biol 6: 402. doi:10.1038/msb.2010.58
Chakrabarty Y, Bhattacharyya SN (2017) Leishmania donovani restricts
mitochondrial dynami cs to enhance mirnp stability and target rna
repression in host macrophages. Mol Biol Cell 28: 2091–2105.
doi:10.1091/mbc.E16-06-0388
Chen L, Yao X, Yao H, Ji Q, Ding G, Liu X (2020) Exosomal mir-103-3p from lps-
activated thp-1 macrophage contributes to the activation of hepatic
stellate cells. FASEB J 34: 5178–5192. doi:10.1096/fj.201902307RRR
Chen Y, Shen A, Rider PJ, Yu Y, Wu K, Mu Y, Hao Q, Liu Y, Gong H, Zhu Y, et al
(2011) A liver-specific microrna binds to a highly conserved rna
sequence of hepatitis b virus and negatively regulates viral gene
expression and replication. FASEB J 25: 4511–4521. doi:10.1096/fj.11-
187781
Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-
Olguin P, Cybulsky MI, Fish JE (2013) Microrna-146 represses
endothelial activation by inhibiting pro-inflammatory pathways.
EMBO Mol Med 5: 1017–1034. doi:10.1002/emmm.201202318
Curtale G, Rubino M, Locati M (2019) Micrornas as molecular switches in
macrophage activation. Front Immunol 10: 799. doi:10.3389/
fimmu.2019.00799
Das S, Mukherjee S, Ali N (2021) Super enhancer-mediated transcription of
mir146a-5p drives m2 polarization during leishmania donovani
infection. PLoS Pathog 17: e1009343. doi:10.1371/journal.ppat.1009343
de Carvalho RVH, Andrade WA, Lima-Junior DS, Dilucca M, de Oliveira CV,
Wang K, Nogueira PM, Rugani JN, Soares RP, Beverley SM, et al (2019)
Leishmania lipophosphoglycan triggers caspase-11 and the non-
canonical activation of the nlrp3 infl
ammasome. Cell
Rep 26:
429–437.e5. doi:10.1016/j.celrep.2018.12.047
De Melo P, Pineros Alvarez AR, Ye X, Blackman A, Alves-Filho JC, Medeiros AI,
Rathmell J, Pua H, Serezani CH (2021) Macrophage-derived microrna-
21 drives overwhelming glycolytic and inflammatory response during
sepsis via repression of the pge2/il-10 axis. J Immunol 207: 902–912.
doi:10.4049/jimmunol.2001251
Fern
´
andez-Messina L, Guti
´
errez-V
´
azquez C, Rivas-Garc
´
ıaE,S
´
anchez-Madrid
F, de la Fuente H (2015) Immunomodulatory role of micrornas
transferred by extracellular vesicles. Biol Cell 107: 61–77. doi:10.1111/
boc.201400081
Fu Y, Zhang L, Zhang F, Tang T, Zhou Q, Feng C, Jin Y, Wu Z (2017) Exosome-
mediated mir-146a transfer suppresses type i interferon response
and facilitates ev71 infection. PLoS Pathog 13: e1006611. doi:10.1371/
journal.ppat.1006611
Ghosh J, Bose M, Roy S, Bhattacharyya SN (2013) Leishmania donovani targets
dicer1 to downregulate mir-122, lower serum cholesterol, and
facilitate murine liver infection. Cell Host Microbe 13: 277–288.
doi:10.1016/j.chom.2013.02.005
Ghosh S, Bose M, Ray A, Bhattacharyya SN (2015) Polysome arrest restricts
mirna turnover by preventing exosomal export of mirna in growth-
retarded mammalian cells. Mol Biol Cell 26: 1072–1083. doi:10.1091/
mbc.E14-11-1521
Ghosh S, Mukherjee K, Chakrabarty Y, Chatterjee S, Ghoshal B, Bhattacharyya
SN (2021) Gw182 proteins restrict extracellular vesicle-mediated
export of micrornas in mamma lian cancer cells. Mol Cell Biol 41:
e00483-20. doi:10.1128/MCB.00483-20
Ghoshal B, B ertrand E, Bhattacharyya SN (2021) Non-canonical argonaute
loading of extracellular vesicle-derived exogenous single-stranded
miRNA in recipient cells. J Cell Sci 134: jcs253914. doi:10.1242/jcs.253914
Gioseffi A, Hamerly T, Van K, Zhang N, Dinglasan RR, Yates PA, Kima PE (2020)
Leishmania-infected macrophages release extracellular vesicles that
can promote lesion development. Life Sci Alliance 3: e202000742.
doi:10.26508/lsa.202000742
Goswami A, Mukherjee K, Mazumder A, Ganguly S, Mukherjee I, Chakrabarti S,
Roy S, Sundar S, Chattopadhyay K, Bhattacharyya SN (2020) Microrna
exporter hur clears the internalized pathogens by promoting pro-
inflammatory response in infected macrophages. EMBO Mol Med 12:
e11011. doi:10.15252/emmm.201911011
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications
of exosomes. Science 367: 6478. doi:10.1126/science.aau6977
Klecker T, B
¨
ockler S, Westermann B (2014) Making connections:
Interorganelle contacts orchestrate mitochondrial behavior. Trends
Cell Biol 24: 537–545. doi:10.1016/j.tcb.2014.04.004
Lenk EJ, Redekop WK, Luyendijk M, Fitzpatrick C, Niessen L, Stolk WA, Tediosi F,
Rijnsburger AJ, Bakker R, Hontelez JAC, et al (2018) Socioeconomic
benefit to individuals of achieving 2020 targets for four neglected
tropical diseases controlled/eliminated by innovative and intensified
disease management: Human african trypanosomiasis, leprosy,
visceral leishmaniasi s, chagas disease. PLoS Negl Trop Dis 12:
e0006250. doi:10.1371/journal.pntd.0006250
Li
´
evin-Le Moal V, Loiseau PM (2016) Leishmania hijacking of the macrophage
intracellular compartments. FEBS J 283: 598–607. doi:10.1111/
febs.13601
Lindsay MA (2008) Micrornas and the immune response. Trends Immunol 29:
343–351. doi:10.1016/j.it.2008.04.004
Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H
(2004) Adiponectin protects lps-induced liver injury through
modulation of tnf-alpha in kk-ay obese mice. Hepatology 40: 177–184.
doi:10.1002/hep.20282
Mazumder A, Bose M, Chakraborty A, Chakrabarti S, Bhattacharyya SN (2013) A
transient reversal of mirna-mediated repression controls
macrophage activation. EMBO Rep 14: 1008–1016. doi:10.1038/
embor.2013.149
Momen-Heravi F, Bala S, Kodys K, Szabo G (2015) Exosomes derived from
alcohol-treated hepatocytes horizontally transfer liver specific
mirna-122 and sensitize monocytes to lps. Sci Rep 5: 9991. doi:10.1038/
srep09991
Mukherjee B, Mukherjee K, Nanda P, Mukhopadhayay R, Ravichandiran V,
Bhattacharyya SN, Roy S (2021) Probing the molecular mechanism of
aggressive infection by antimony resistant leishmania donovani.
Cytokine 145: 155245. doi:10.1016/j.cyto.2020.155245
Mukherjee B, Mukhopadhyay R, Bannerjee B, Chowdhury S, Mukherjee S,
Naskar K, Allam US, Chakravortty D, Sundar S, Dujardin JC, et al (2013)
Antimony-resistant but not antimony-sensitive leishmania donovani
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 22 of 23

up-regulates host il-10 to overexpress multidrug-resistant protein 1.
Proc Natl Acad Sci U S A 110: E575 –E582. doi:10.1073/pnas.1213839110
Mukherjee K, Ghoshal B, Ghosh S, Chakrabarty Y, Shwetha S, Das S,
Bhattacharyya SN (2016) Reversible hur-microrna binding controls
extracellular export of mir-122 and augments stress response. EMBO
Rep 17: 1184–1203. doi:10.15252/embr.201541930
O’Connell RM, Rao DS, Baltimore D (2012) Microrna regulation of
inflammatory responses. Annu Rev Immunol 30: 295–312. doi:10.1146/
annurev-immunol-020711-075013
Ordas A, Kanwal Z, Lindenberg V, Rougeot J, Mink M, Spaink HP, Meijer AH
(2013) Microrna-146 function in the innate immune transcriptome
response of zebrafish embryos to salmonella typhimurium infection.
BMC Genomics 14: 696. doi:10.1186/1471-2164-14-696
Poria DK, Guha A, Nandi I, Ray PS (2016) Rna-binding protein hur sequesters
microrna-21 to prevent translation repression of proinflammatory
tumor suppressor gene programmed cell death 4. Oncogene 35:
1703–1715. doi:10.1038/onc.2015.235
Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G,
Goukassian D, Zhu Y, Losordo DW, et al (2006) Il-10-induced tnf-alpha
mrna destabilization is mediated via il-10 suppression of p38 map
kinase activation and inhibition of hur expression. FASEB J 20:
2112–2114. doi:10.1096/fj.06-6084fje
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M,
Bursac D, Angrisano F, Gee M, Hill AF, et al (2013) Cell-cell
communication between malaria-infected red blood cells via
exosome-like vesicles. Cell 153: 1120–1133. doi:10.1016/j.cell.2013.04.029
RexJ,LutzA,FalettiLE,AlbrechtU,ThomasM,BodeJG,BornerC,SawodnyO,
Merfort I (2019) IL-1β and TNFα different ially influence NF-κB activity and
FasL-induced apoptosis in primary murine hepatocytes during LPS-
induced inflammation. Front Physiol 10: 117. doi:10.3389/fphys.2019.00117
Rupaimoole R, Calin GA, Lopez-B erestein G, Sood AK (2016) Mirna
deregulation in cancer cells and the tumor microenvironment. Cancer
Discov 6: 235–246. doi:10.1158/2159-8290.CD-15-0893
Scianimanico S, Desrosiers M, Dermi ne JF, M
´
eresse S, Descoteaux A,
Desjardins M (1999) Impaired recruitment of the small gtpase rab7
correlates with the inhibition of phagosome maturation by
leishmania donovani promastigotes. Cell Microbiol 1: 19–32.
doi:10.1046/j.1462-5822.1999.00002.x
Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ,
Reiner NE (2010) An exosome-based secretion pathway is responsible
for protein export from leishmania and communication with
macrophages. J Cell Sci
123: 842–852. doi:10.1242/jcs.056465
Srikantan S, Gorospe M (2012) Hur function in disease. Front Biosci
(Landmark Ed) 17: 189–205. doi:10.2741/3921
Sunter J, Gull K (2017) Shape, form, function and leishmania pathogenicity:
From textbook descriptions to biological understanding. Open Biol 7:
170165. doi:10.1098/rsob.170165
Testa U, Pelosi E, Castelli G, Labbaye C (2017) Mir-146 and mir-155: Two key
modulators of immune response and tumor development. Noncoding
RNA 3: 22. doi:10.3390/ncrna3030022
Th
´
ery C, Zitvogel L, Amigorena S (2002) Exosomes: Composition, biogenesis
and function. Nat Rev Immunol 2: 569–579. doi:10.1038/nri855
Todkar K, Chikhi L, Germain M (2019) Mitochondrial interaction with the
endosomal compartment in endocytosis and mitochondrial
transfer. Mitochondrion 49: 284–288. doi :10.1016/
j.mito.2019.05.003
Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR (2014)
Mechanisms of cellular invasion by intracellular parasites. Cell Mol
Life Sci 71: 1245–1263. doi:10.1007/s00018-013-1491-1x
Wang H, Xu W, Shao Q, Ding Q (2017) Mir-21 silencing ameliorates
experimental autoimmune encephalomyelitis by promoting the
differentiation of il-10-producing b cells. Oncotarget 8: 94069–94079.
doi:10.18632/oncotarget.21578
License: This article is available under a Creative
Commons License (Attribution 4.0 International, as
described at https://creativecommons.org/
licenses/by/4.0/).
Leishmania hijacks host miRNA machinery Ganguly et al. https://doi.org/10.26508/lsa.202101229 vol 5 | no 6 | e202101229 23 of 23
