
ClientName:
Patient’sName:
ExamDate:
Japan Health Certificate Package
I. Instructions:
(1) Printpages2‐3.Giveblankhardcopiestoclientswhentheynotifyyouofupcomingtravel.
(2) Saveanewcopyofthisfileforeachpet.
(3) ClientfillsouttopofPage3asmuchastheycan,clinictranscribestodigi talfilepage3.
(4) Printpg4‐5forclientstosubmitAdvancedNotifi cation(notrequiredformil itaryflights).
(5) Printautopopulatingpages6‐10forHealthCertificateappointment.
(6) Compileadditionalrequireddocum ents–2rabiescertificates&FAVN.
(7) VCO/VMOsign&stampwhereappropriate.DONOTSIGNINBLACK!(typicallyblue).
(8) Printcoverpageandusechecklistatbottomduringfinalreviewtoensureallrequired
documentsarepresent.
II. Special Notes and Helpful Hints:
(1) Filloutallblocksonpage3,eveniftheyseemredundant.
(2) Dateisalmostalwaysneededintwoformats,YYYY/MM/DD&d d/mm/yyyy.The
documentwillpromptyoutousethecorrectformatasneeded.
(3) Onlyonepetperpacket.
(4) Ifpethasmorethanonemicrochip,listBOTHmicrochipnumbers.
(5) ThispacketisforusebyVCOsandGS VMOsonly.NAFVMOsarenotauthorizedtousethe
MDJOPForm2209norsignbothsidesoftheAPHIS7001.
(6) Thesummarysheetdescribestheabsoluteminimumtimelineneededtomeetentry
requirements.Useclinicaljudgm entwhenestablishingtreatmentplanstoensuregreatest
chanceofpassingFAVN(e.g.don’tdrawFAVNonsamedayasrabiesvaccinationunless
absolutelynecessaryduetotraveltimerestrictions).
(7) Alloriginalformsmustbesignedinacolorotherthanblack(typicallyblue).
(8) Attheirdiscretion,aVCOcancountersignasignedcopyofarabiescertificatefromany
otherveterinarian(militaryorcivilian)tocertifyitasvalidanditisthenconsideredan
“original”.
III. ChecklistforuseondayofHealthCertificateexam:
(1) Originalrabiescertificate#1
(2) Originalrabiescertificate#2
(3) OriginalFAVN
(4) USDAAphis7001
(5) MDJOPForm2209
(6) JapanFormA/C
(7) Acclimationmemo
(8) USDAsignatureauthoritymemo
FinalVersion–14January 2019

Japan Requirements Summary
Thissheetisasummaryonlyandnotacomprehensiveguide.Itisthepetowner’sresponsibilitytoverifyinformationonofficial
USDAandJapanreferences.ImportantinfocanbefoundinJapan’s“GuidetoimportingdogsandcatsintoJapanfromNon‐
designatedregions.”Alsovisit:http://www.usarj.army.mil/organization/vet/import.aspx
1. Microchip
Ideally,administeredPRIOR(samedayok)to1
st
rabiesvaccine.
Maymicrochipafter1stRabiesandbefore(samedayok)2
nd
RabiesbutcausesstricttimelinerestrictionsonFAVN
sampledraw.SeeFAVNBloodTestbelowfordetails.
Shouldbe15‐digitISOcompatible.IfnotISOcompatible,eitheraskdestinationVTFiftheyhaveacompatiblereader
forcurrentchip(mostdo),haveownerbuytheirownreader,orre‐microchipandstartvaccinationprocessover.
2. 1
st
RabiesVaccine
Petmustbeatleast91daysold.
FelinePureVaxisacceptedatalllocationsasofJanuary2019.
3. 2
nd
RabiesVaccine
Mustbe>31daysafter1
st
vaccinationandBEFORE1
st
vaccineexpires.
Japanwillhonor3yearrabiesforentrypurposes.Afterarrival,allpetsmustbevaccinatedannually.
4. FAVNBloodTest
IfmicrochippedBEFORE1
st
rabies,FAVNcanbedrawnsamedayorafter2
nd
rabiesvaccine.
IfmicrochippedAFTER1
st
rabies,FAVNMUSTbedrawnsamedayas2
nd
rabiesvaccine.
FAVNmustbe>to0.5IU/ml.
Resultsaresentdirectlytoowner’saddressonFAVNsubmissionform,typicallyautopopulatedfromaddresson
ROVR.VERIFYADDRESSPRIORTOSUBMISSION.Ownermustmaintainoriginalfortravel.
5. AdditionalRabiesVaccines:
If2
nd
rabieswillexpirepriortoarrival,boosterasneeded,butlistonly1
st
and2
nd
rabiesonJapanformsA/CandMDJ
OP6/DDForm2209.
Travelwithoriginalrabiescertificateof3
rd
rabiesvaccinetoprovecurrentvaccinestatus.
6. AdditionalVaccinesandTreatments:
Thefollowingarestronglyrecommendedduetovaryingrequirementsinlodging,kennels,andquarantines:
DAP/FVRCP,Bordetella,andLeptovaccines,andinternal/externalparasitetreatments.
7. AdvanceNotification
AfterownerreceivesFAVNresultsandnotlessthan40daysbeforearrival,ownermustsubmita“Notification”to
AnimalQuarantineService(AQS)attheexpectedportofentry(seep.23ofGuide)
Alldataon“Notification”MUSTmatchdataonhealthcertificatesorquarantinewilllikelybeimposed.
AdvancenotificationNOTrequiredfordirectmilitaryflightsonbase,butownerisSTRONGLYadvisedtocontact
veterinarytreatmentfacility(VTF)onbaseoffinaldestinationpriortotravel.
o Contactinformationcanbefoundat:http://www.usarj.army.mil/organization/vet/contacts.aspx
8. 180 Day Waiting Period after FAVN is Drawn
Ifpetarrivesduringwaitingperiod,petwillbequarantinedfordurationofquarantineassignedatentry.
SOFAPersonnel:within72hoursafterarrival,presentpettoVTFforregistrationandquarantineexam.
o Ifnoquarantinedassignedatentry,petwillbeofficiallyreleased.
o Ifquarantineassigned,petmustremainon‐base(inowner’scustody)oratapprovedon‐basepetboarding
facility(atowner’sexpense).Atlimitedlocations,fosteringbyfamiliesmaybeavailable.
9. FinalVeterinaryAppointment
Nomorethan10dayspriortoarrivalinJapan,receiveveterinaryexamandhealthcertificatedocuments.

DataImportSheet‐Japan
Dearpetowner,filloutthetopsectiontothebestofyourabilityandreturntotheclinicpreparingthehealthcertificate.
Coordinatewithveterinaryclinictoensurepaperworkisreturnedwithsufficienttimepriortohealthcertificateappointment.
SectionI–PetOwnertoProvide
ClientInfo–useaddressonordersifpossible
Consignor/Shipper(Last,First):________________________
AddressLine1_________________________________
AddressLine2_________________________________
Phone:______________________________________
Consignee/Recipient(Last,First):_______________________
AddressLine1_________________________________
AddressLine2_________________________________
Phone:______________________________________
CountryofExport:_____________________
PetInfo:
PetName:_______________;Tattoo:______n/a
Breed:___________________Color:___________________
Sex(M/F/MN/FS):______Sex:MaleFemale;
Age:_____;Age:3‐12months>12months
DOB:__________&____________(2formats)
MicrochipType:_____________Brand:____________
ImplantDate:___________&____________(2formats)
Microchip#:__________________________________
Size:<20lbs20‐50lbs>50lbs
Use:Pet;Other:____________
SectionII–PetOwnertoProvide.NeededonlyforAdvancedNotification,skipifdirectmilitaryflight.
DepartureDate&Location:___________________________
ArrivalDate:_________Port/Airport:___________________
NameofVesselorFlight#:____________________________
CountriesPetvisitedinpast12months&dateofvisits:
_________________________________________________
Length:______cm;Height:_______cm;Weight:_______kg
Transportmethod:__________________(HandLuggage=in
cabinorcheckedbaggage;Cargo=manifestcargo)
MicrochipSite:__________Shipper’sFax#:______________
Shipperemailaddress:_______________________________
SectionIII–ClinicUseOnly‐patientspecific
RabiesVaccine#1(mostrecent)
1yr3yr;Type:KilledModified;Tag#:____
Date:_________&________Duration:____year(s)
Manufctr:_____ProductName:____________Batch#:_____
BoosterDue:________VialExpires:________Type:________
RabiesVaccine#2(older):
1yr3yr;Type:KilledRecombinant
Date:_________&________Duration:____year(s)
Manufctr:_____ProductName:____________Batch#:_____
BoosterDue:________VialExpires:________Type:________
FAVN:
SerumDrawDate:_____________&_____________
DVMName:______________ClinicName:_______________
AddressLine1________________________________
AddressLine2_________________________________
LabPerformingFAVN:________________________________
LabAddress:_______________________________________
LabRegistration#(leaveblankforFADL):________________
TestResults>________IU/ml
RecommendedAdditionalVax/Tx’s:
DA2PPL‐Cvk/FVRCCP;(dd/mm/yyyy):______________
*Thischeckboxisfr omMDJOPForm2209,useatDVM’sdiscret ion
VACCINE#1:
Duration:_____________Type:___________
Date:_________BoosterDueDate:__________________
Product&Manufacturer:___________________________
VACCINE#2:
Duration:_____________Type:___________
Date:_________BoosterDueDate:__________________
Product&Manufacturer:___________________________
EXTERNALPARASITETREATMENT:
Date:_________
Type&Manufacturer:______________________________
INTERNALPARASITETREATMENT:
Date:_________
Type&Manufacturer:______________________________
SectionIV–ClinicUseOnly‐clinicspecific
VeterinarianInfo:
SigningDVMRank&Name:___________________________
OfficialPosition:_____________________________
State&License:___________Accreditation#:____________
ClinicName:_______________________________________
AddressLine1:_______________________________
AddressLine2:_______________________________
PhoneNumber:______________________________
Miscellaneous:
Examdate:______&_______&________
APHISCert#:_______________________________________
OfficeSymbolforMFR:_______________________________
2018/12/13
DOD
Veterinary Food Analysis and Diagnostic
Laboratory
.
.
.
.
.
.
.
.
.
.
.
.
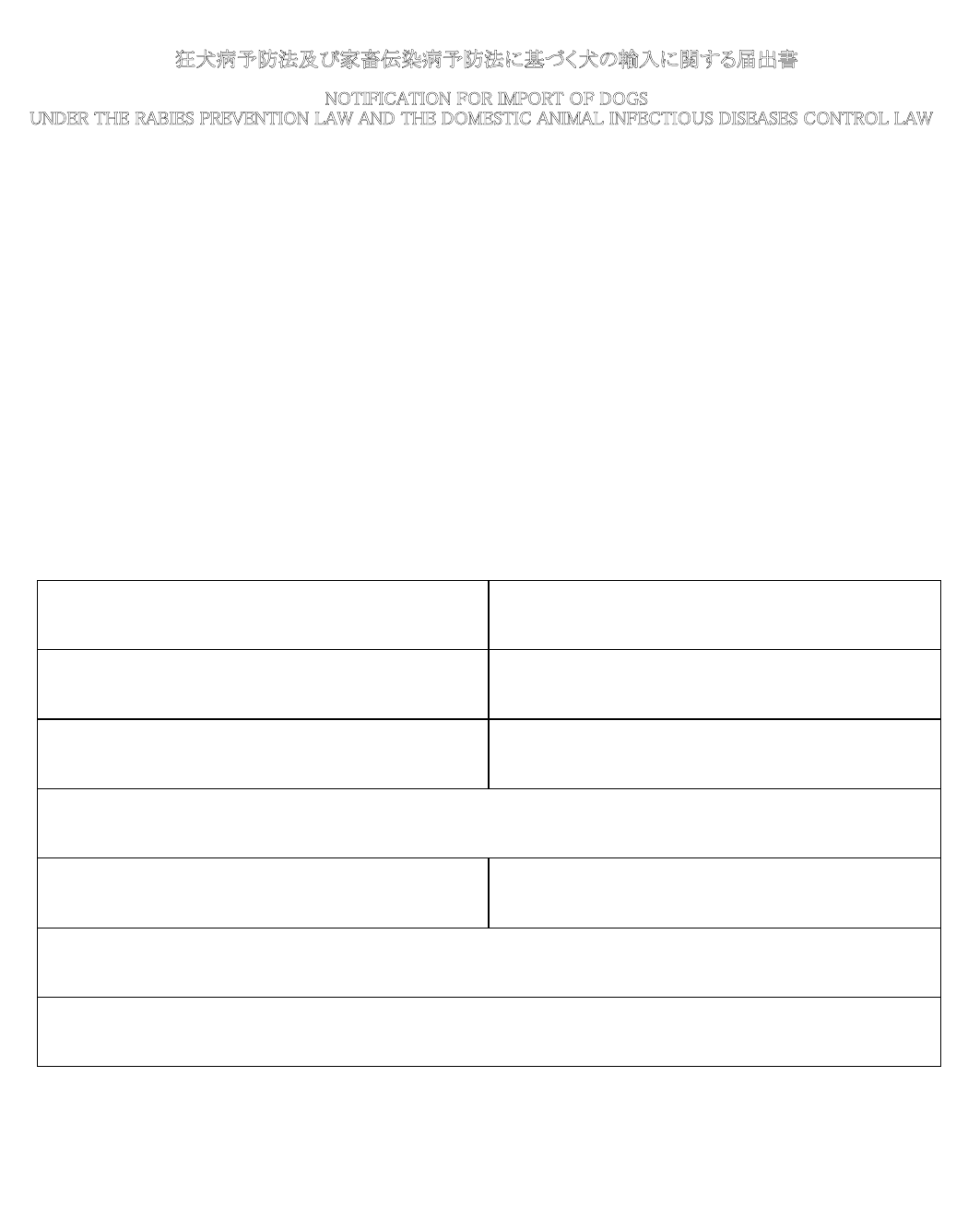
Name and address of applicant
Name
Address
Telephone
FAX
E-mail
Species of animal(s) Quantity
Date of birth (Age) Sex
Country of export Scheduled place of arrival
Scheduled date and place of embarkation
Scheduled date of arrival (year/month/day) Name of scheduled vessel (or flight No.)
Name and address of consignor
Name and address of consignee
2.
To the chief of Animal Quarantine Service
YearMonth Day
I hereby notify for the importation of the undermentioned animal(s).
In the last column of next page, please note the information such as the use of the animal(s), the destination, name and address of the
facility in which the animal(s) is/are kept, etc.
Canis familiaris
(Dog)
One (1)
2018/12/13

(Other useful information)
Name of animal(s)
/
Means for identification (e.g.microchip) Identification number/Mark
Date of identification(year/month/day) Location of identification Type of microchip (reader)
Breed Color
Use cargo or hand luggage
Length
Height
Weight
Name and address of the facility in which the animal(s) is/are kept
Name and address of destination
Countries visited in the past 12 months and the date of visits
Date of blood sampling (year/month/day) Antibody titer
IU/ml
Name and address of the designated laboratory
Remarks
Date of vaccination
(year/month/day)
Date of expiry
(year/month/day)
Kind of vaccine
Other vaccination
Name of product and manufacturer
Rabies serological test
Name of product and manufacturer
Before blood sampling
Date of vaccination
(year/month/day)
Date of expiry
(year/month/day)
Rabies vaccination
Booster(if any)
Kind of vaccine
Microchip
Not Applicable
`
Pet
Other: ________________
.
.
.
.
DOD Veterinary Food Analysis and Diagnostic Laboratory
.
.

DEPARTMENT OF THE ARMY
PUBLIC HEALTH ACTIVITY
REPLY TO
MEMORANDUM FOR COMMERCIAL AIRLINES
SUBJECT: Temperature Tolerance for a Dog / Cat - Acclimation Statement
1. The below listed animal(s) in this shipment appears healthy for transport but needs to
be maintained at a temperature within the animal’s thermoneutral zone.
2. The temperatures that the animal is exposed to while inside a terminal facility must
not be lower than 45 degrees Fahrenheit (45ºF) for more than 4 consecutive hours, nor
lower than 45 degrees Fahrenheit (45ºF) for more than 30 minutes when moving the
animal from terminal facilities or primary conveyances. The animal should not be
subjected to temperatures lower than 30 degrees Fahrenheit (30ºF) for more than 15
minutes.
3. Auxiliary ventilation, such as fans, blowers, or air conditioning, must be used during
surface transportation in any animal cargo space containing live animals when the
ambient temperature exceeds 85 degrees Fahrenheit (85ºF). Moreover, the ambient
temperature may not exceed 85 degrees Fahrenheit (85ºF) for a period of more than 4
consecutive hours. These temperatures are in accordance with Title 9 Code of Federal
Regulations.
Consignor Name: _____________________
Consignor Address: _____________________
_____________________
Pet Name: Microchip: Species: Color: Gender: Breed:
Signature:
Stamp:
Canis familiaris
(Dog)

According to t he Paperwork R eduction Act o f 1995, an ag ency may not cond uct or sponsor, and a p erson is not required to respond to, a c ollection of
information unless it displays a valid OMB control number. The valid OMB control numbers f or this information collection are 0579-0036 and 0579-0333.
The ti me r equired to complete t his i nformation col lection i s esti mated t o av erage .25 h ours per r esponse, i ncluding the ti me for rev iewing in structions,
searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information.
No dog, cat, nonhuman primate, or additional kinds or classes of animals designated by
USDA r egulation sh all be del ivered to any i ntermediate handler or car rier for
transportation i n com merce, u nless accompanied by a heal th ce rtificate ex ecuted a nd
issued by a licensed veterinarian (7 U.S.C. 21.43.9; CFR, Subchapter A, Part 2).
OMB APPROVED
0579-0036
0579-0333
UNITED STATES DEPARTMENT OF AGRICULTURE
ANIMAL AND PLANT HEALTH INSPECTION SERVICE
UNITED STATES INTERSTATE AND INTERNATIONAL
CERTIFICATE OF HEALTH EXAMINATION
FOR SMALL ANIMALS
WARNING: Anyone who makes
a false, fictitious, or fraudulent
statement on this document, or
uses such document knowing it
to be false, fictitious, or
fraudulent may be subject to a
fine of not more than $10,000 or
imprisonment of not more than 5
years or both (18 U.S.C. 1001).
1. TYPE OF ANIMAL SHIPPED (select one only)
Dog Cat Other_________________
Nonhuman Primate Ferret
Rodent
2. CERTIFICATE NUMBER - OFFICIAL USE ONLY
3. TOTAL NUMBER OF ANIMALS 4. PAGE
5. NAME, ADDRESS, AND TELEPHONE NUMBER OF OWNER (C
O
N
S
IG
N
O
R)
USDA License/or Registration Number (i
f app
lic
ab
l
e
)
6. NAME, ADDRESS, AND TELEPHONE NUMBER OF RECIPIENT AT DESTINATION (C
O
N
SIG
N
EE
)
7. ANIMAL IDENTIFICATION 8. PERTINENT VACCINATION, TREATMENT, AND TESTING HISTORY
NAME, AND/OR TATTOO NUMBER
OR OTHER IDENTIFICATION
BREED – COMMON
OR SCIENTIFIC
NAME
AGE SEX
COLOR OR
DISTINCTIVE
MARKS OR
MICROCHIP
RABIES VACCINATION
1 YEAR 2 YEARS 3 YEARS
OTHER VACCINATIONS,
TR
EATMENT, AND/OR TESTS AND RESULTS
Vaccination Date Product Date Product Type and/or Results
(1)
(2)
(3)
(4)
(5)
(6)
9. REMARKS OR ADDITIONAL CERTIFICATION STATEMENTS (WH
E
N
R
EQ
U
I
R
E
D)
VETERINARY CERTIFICATION: I certify that the animals described in box 7 have been examined by me this date, that the
information provided in box 8 is true and accurate to the best of my knowledge, and that the following findings have been made
(“X” applicable statements).
I have verified the presence of the microchip, if a microchip is listed in box 7.
I certify that the animal(s) described above and on continuation sheet(s), if applicable, have been inspected by me on this date and
appear to be free of any infectious or contagious diseases and to the best of my knowledge, exposure thereto, which would endanger the
animal or other animals or would endanger public health.
To my knowledge, the animal(s) described above and on continuation sheet(s) if applicable, originated from an area not quarantined
for rabies and has/have not been exposed to rabies.
ENDORSEMENT FOR INTERNATIONAL EXPORT (
I
F
N
EE
D
E
D) NAME, ADDRESS, AND TELEPHONE NUMBER OF ISSUING VETERINARIAN LICENSE NUMBER AND STATE
PRINTED NAME OF USDA VETERINARIAN
Accredited Yes No
If yes, please complete below
NATIONAL ACCREDITATION NUMBER
DATE DATE
APHIS Form 7001
(NOV 2010) This certificate is valid for 30 days after issuance
1 of 1
x
x
x
x
One (1)
Date FAVN Drawn:
Results>
IU/mL
Lab Name:
X
Note: All dates, unless otherwise noted, are in the format YYYY/MM/DD
DOD Veterinary Food Analysis and Diagnostic Laboratory
.
.

VETERINARY HEALTH CERTIFICATE
For Import/Export for Japan
THIS FORM IS SUBJECT TO THE PRIVACY ACT OF 1974
AUTHORITY: 10 U.S.C. Sections 133 and 8012.
PRINCIPAL PURPOSE(S): To indicate general health examination of the animal to permit international movement.
ROUTINE USE(S): Used as health certificate to permit international movement of animal.
DISCLOSURE: Providing personal information is voluntary. However, if information is not disclosed by the owner, international
movement may not be allowed.
INFORMATIO N OF OWNER
T
ype of Print Name of O
w
n
e
r
(
Last, Fist, MI):
T
elephone Number:
C
omplete Address (Include Zip Code
)
D
ESCRIPTION OF ANIM
AL
Name of Animal:
S
p
e
c
i
e
s:
Dog Cat ( )
T
ag Number:
Predominant Breed:
C
olor(s):
Sex:
Male
Female
Age: 3 – 12 months
12 months or older
Date of Birth (dd/mm/yyyy):
Weight: Under 20 lbs
20 – 50 lbs
Over 50 lbs
RABIES IMMUNIZ
A
TION DAT
A
MICROCHIP/IDENTIFICATION DAT
A
Rabies Vaccine History Most Recent Prior
Implantation Date
(dd/mmm/yy)
Producer (First 3 letters)
Rabies Vaccine Name
Microchip Number
Vaccine Effective Period
1 Year 3 Years 1 Year 3 Years
Vaccine Type
Killed Recombinant Killed Recombinant
Manufacturer of
Microchip
Lot/Serial Number
Vaccination Date (d/m/y)
Tattoo Number
N/A
Vaccine Expiration Date
Other Vaccinations: DA2PPL-Cvk / FVRCCP Date:
FLUORESCENT ANTIBODY VIRAL NEUTRALIZATION TEST(
S
) (FAVN
)
Date of
S
ampling (dd/mm/yyyy)
:
V
eterinarian Name and Address
:
T
est Results (
≥
0.5 IU/ml
)
:
IU/ml
L
aborator
y
Name and Re
g
istration Number:
T
his is to certify that the above described animal has been examined by me on the date below and was found free of
any communicable disease. To the best of my knowledge this animal has not been exposed to rabies and did not
originate from a rabies quarantine area.
V
eterinarian Name, Grade, Unit and State License Number (Include state abbreviation and number):
S
i
g
nature Date
DD FORM 2209, APR 2009 Previous edition may be used MDJ O P 6, J AN 2016
State License:
USDA Accreditation#:
X
>
DOD Veterinary Food Analysis and Diagnostic
Laboratory
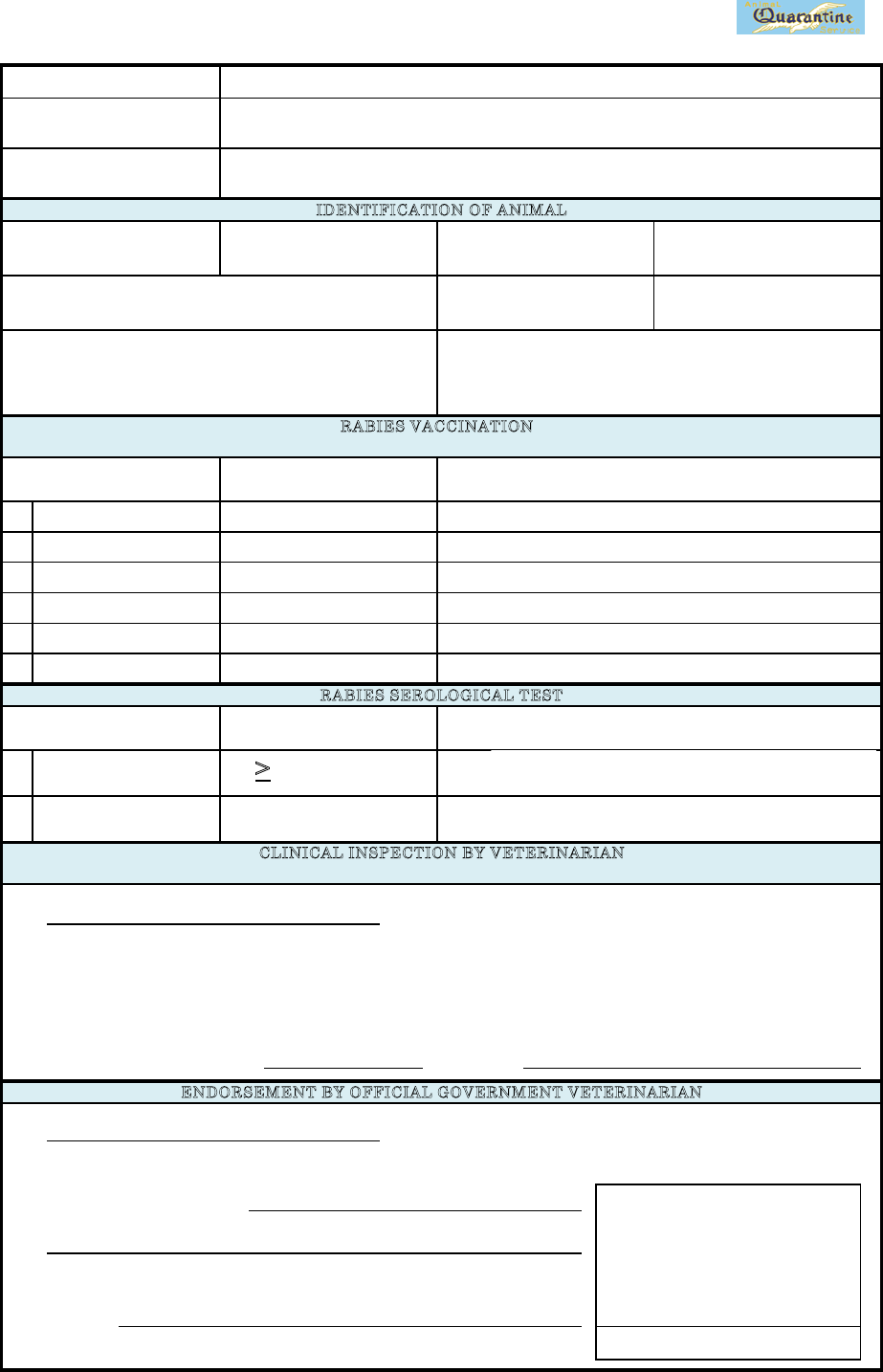
Date (yyyy/mm/dd):
OFFICIAL GOVERNMENT STAMP
that to the best of my knowledge and belief all the details mentioned above are true and correct.
Signature:
ENDORSEMENT BY OFFICIAL GOVERNMENT VETERINARIAN
Signature:
Address of veterinarian:
Date of inspection (yyyy/mm/dd):
C
LINICAL INSPECTION BY VETERINARIA
N
*Immediately before embarkation ( Inspection within 10 days is acceptable )
・
I
have read the microchi
p
im
p
lanted in the animal and confirmed the number.
・
T
he animal has shown no clinical si
g
ns of rabies (and l
ep
tos
p
irosis onl
y
for d
og
).
Country :
Country :
Name :
Certi
f
icate
f
or
dogs
, cats
, f
oxes, raccoons, or skunks to be im
por
ted int
o Japan
Date of vaccination
(yyyy/mm/dd)
Vaccine effective period
(year)
I,
I,
Name and address of office:
Microchip number
Date of identification (yyyy/mm/dd)
Date of birth (yyyy/mm/dd) or Age
Name :
Use
, an official government veterinarian of exporting country certify
Name of product and manufacturer
*Type of vaccine should be inactivated or recombinant
from NON-DESIGNATED REGION
Either type or write clearly in BLOCK letters in English. Do not use pencils or erasable ink to fill in.
No correction fluid shall be used. The original entry shall be struck through and remain legible.
The correction shall be written adjacent to the original and signed.
Species
Breed
Name Sex
IDENTIFICATION OF ANIMAL
Exporting country
Consignor
Name :
Address :
Name :
Address :
Consignee
Form AC
, a veterinarian certify that;
Ⅰ
Ⅱ
RABIES SEROLOGICAL TEST
Date of blood drawing
(yyyy/mm/dd)
Antibody titer
(IU/ml)
The designated laboratory
year(s)
Ⅵ
別記様式第4号の3
year(s)
year(s)
Ⅳ
Ⅴ
Ⅲ
year(s)
□ Pet □ Other:
Color
□ Male □ Female
R
ABIES VACCINATIO
N
*Please write from latest one
(produced in accordance with OIE standard)
Ⅰ
Ⅱ
>
IU/ml
United States of America
year(s)
year(s)
Canis familiaris
(Dog)
.
.
DOD Veterinary Food Analysis and Diagnostic Laboratory
.
.

